Unit 3 Chemical Bonding and Nomenclature Part 1
Unit 3: Chemical Bonding and Nomenclature Part 1 CH 1120
States of Matter � Solid ◦ Definite shape and volume ◦ Cannot be compressed � Liquid ◦ Definite volume but takes shape of container � Gas ◦ No fixed volume or shape ◦ Uniformly fills container
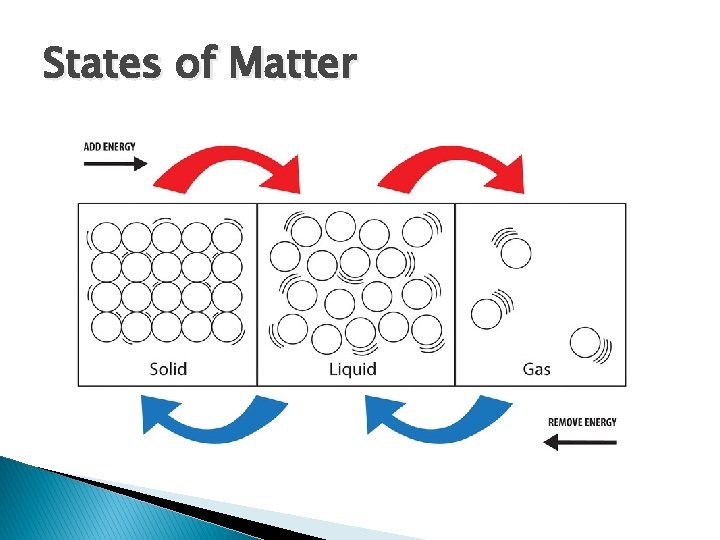
States of Matter
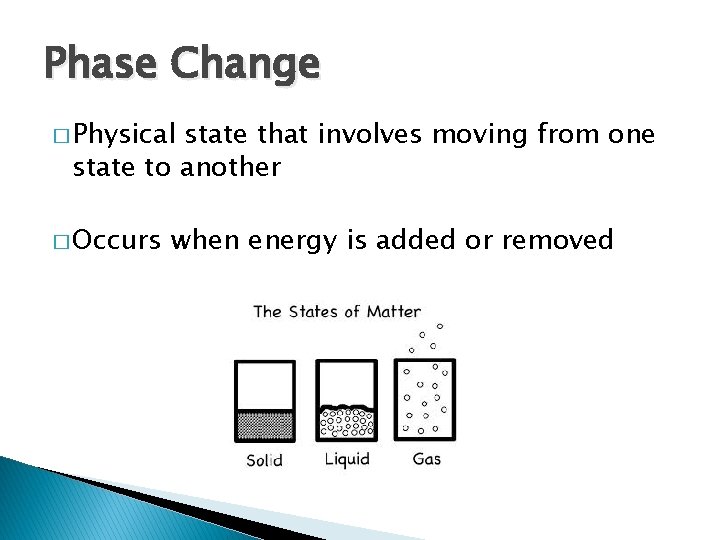
Phase Change � Physical state that involves moving from one state to another � Occurs when energy is added or removed
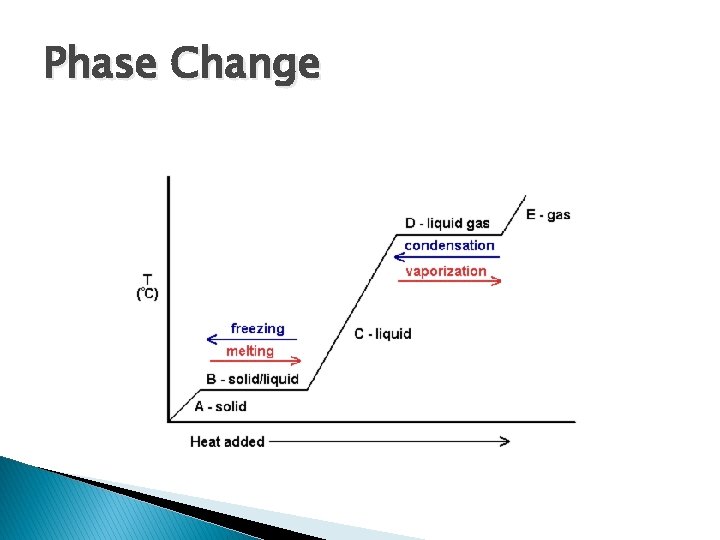
Phase Change
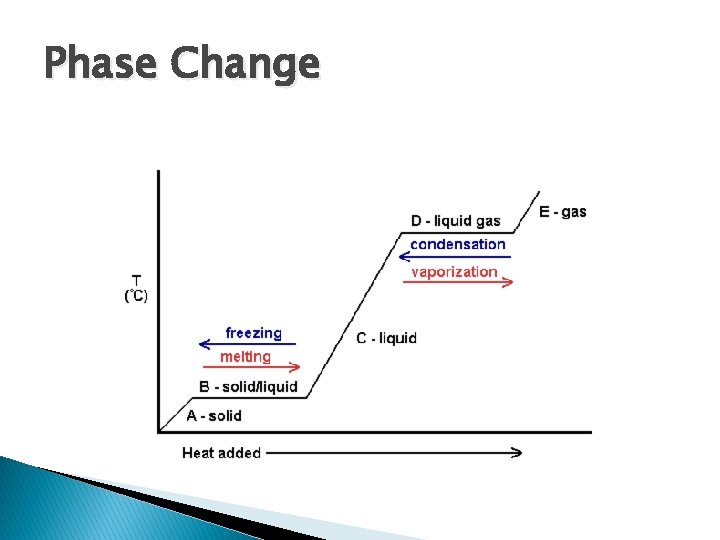
Phase Change � Melting: phase change from solid to liquid � Freezing: phase change from liquid to solid � Temperature where liquid and solid phases coexist at equilibrium is: ◦ Melting point of the solid ◦ Freezing point of the liquid

Phase Change � Vapour: � Vapour gas pressure: pressure exert by a gas on its container when it is at equilibrium with its condensed phases (solid or liquid)
Phase Change � Boiling: the process of molecules in the liquid phase breaking apart from neighbouring molecules to enter the gas phase � Boiling point: temperature when a liquid’s vapour pressure equals the external pressure acting on the liquid surface
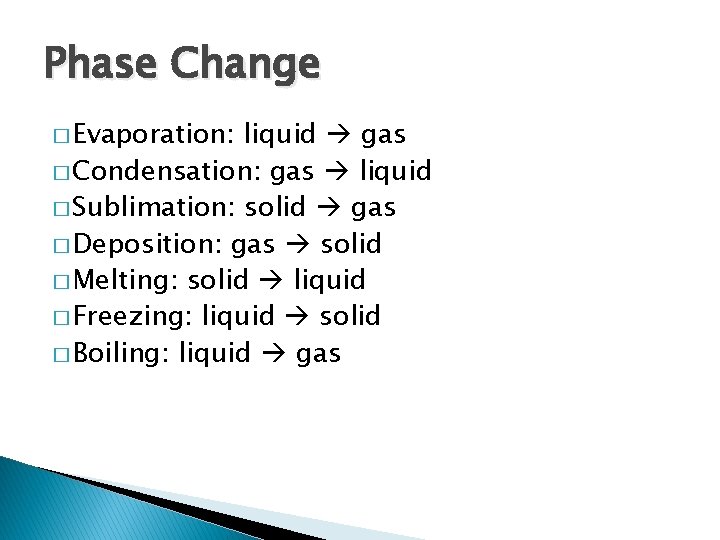
Phase Change � Evaporation: liquid gas � Condensation: gas liquid � Sublimation: solid gas � Deposition: gas solid � Melting: solid liquid � Freezing: liquid solid � Boiling: liquid gas
Phase Change and Kinetic Molecular Theory � Temperature is a measure of the average kinetic energy of the molecules of a substance � Adding energy heats up substances � Energy causes more movement of molecules � Altering molecular movement alters the state of substances
Phase Change
Phase Change � Remember, molecules do not lose their structure when they undergo a phase change ◦ H 2 O is still H 2 O �Steam, water, ice � Molecules them simply have more space between
Octet Rule � Atoms bond together to obtain a stable electron configuration � Atoms gain, lose, or share electrons until they are surrounded by eight valence electrons � Some elements require 2 valence electrons (not 8)
Octet Rule � Think of your orbital diagrams ◦ Elements want to look like the closest noble gas � We use Lewis dot diagrams to show valence electrons and help us see how bonding occurs
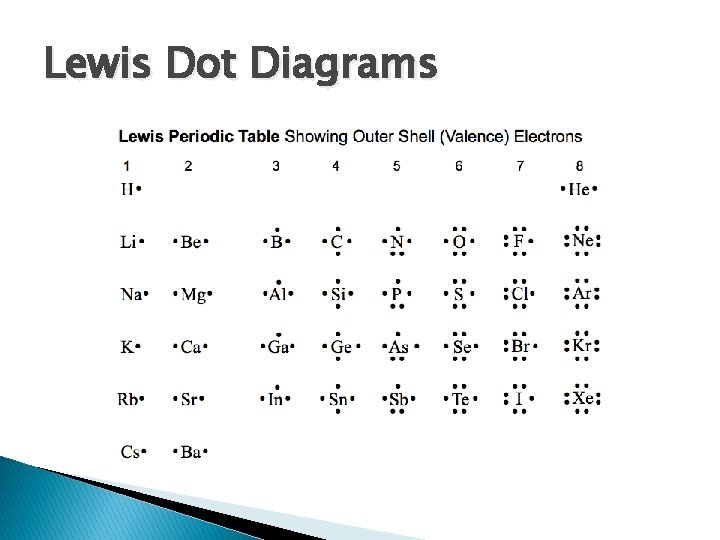
Lewis Dot Diagrams
Lewis Dot Diagrams � Remember, electrons repel each other (negative charge) ◦ They don’t want to fill the same orbital if it can be avoided
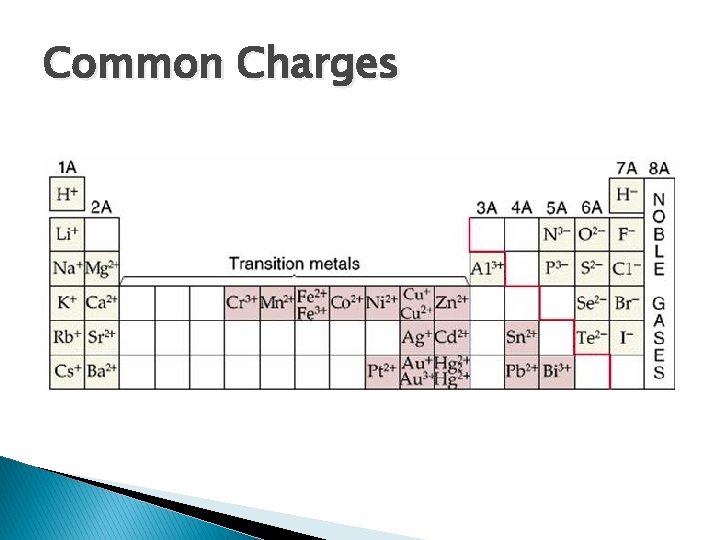
Common Charges

Remember � Electrons are negative ◦ Gaining electrons makes charge more negative ◦ Losing electrons makes charge more positive � Main group elements are lazy and want to look like the closest noble gas � Metals want to lose electrons � Non-metals want to gain electrons
Ionic Vs. Covalent Bonds � In ionic bonding atoms gain and lose electrons ◦ Charge (ions) � In covalent bonding atoms share electrons ◦ No charge (atoms)
Ionic Vs. Covalent Bonds � Ionic bonds contain a metal and one or more non-metals � Covalent bonds contain only non-metals
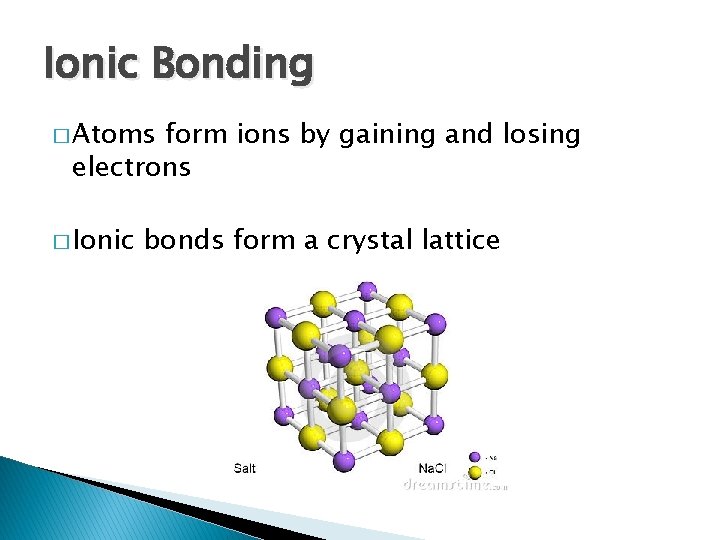
Ionic Bonding � Atoms form ions by gaining and losing electrons � Ionic bonds form a crystal lattice
Ionic Compounds � Contain a metal and one or more non-metals � Contain ions (charged atoms) due to transfer of electrons � NO sharing electrons
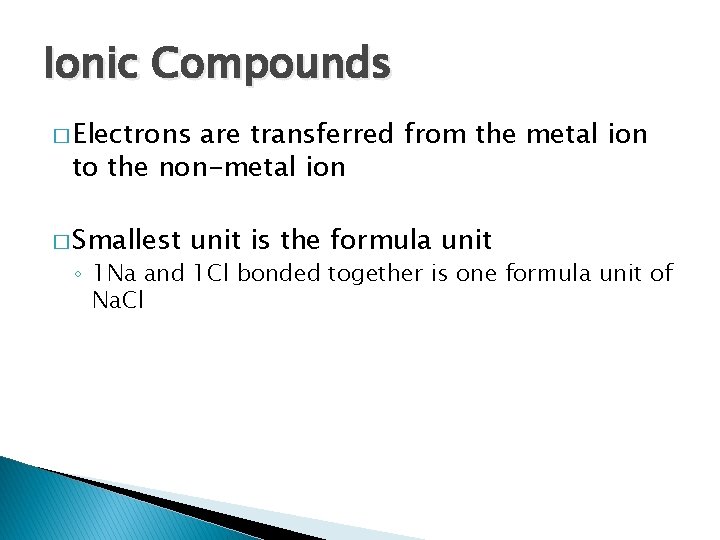
Ionic Compounds � Electrons are transferred from the metal ion to the non-metal ion � Smallest unit is the formula unit ◦ 1 Na and 1 Cl bonded together is one formula unit of Na. Cl

Ionic Compounds � Very high melting and boiling points � Crystalline and can be cleaved ◦ Broken along smooth flat surfaces � Brittle � Conduct electricity when dissolved ◦ Break into ions
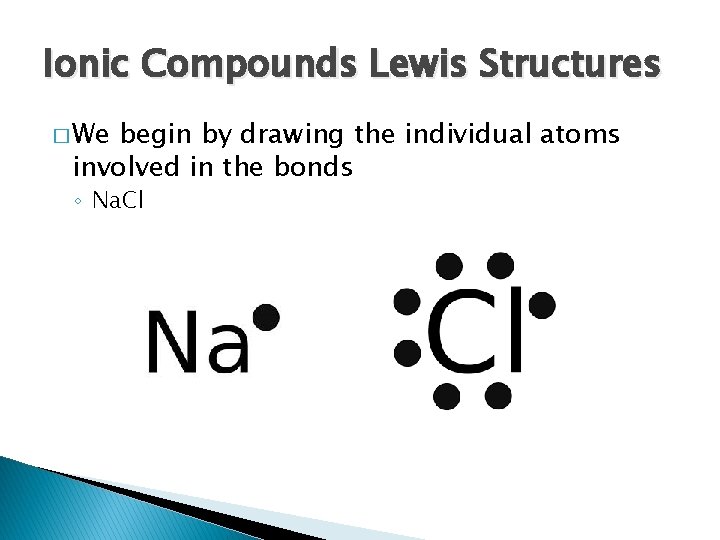
Ionic Compounds Lewis Structures � We begin by drawing the individual atoms involved in the bonds ◦ Na. Cl
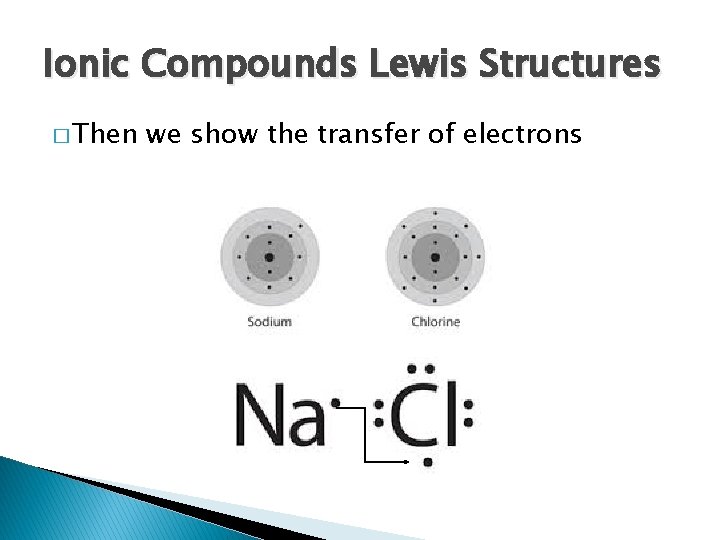
Ionic Compounds Lewis Structures � Then we show the transfer of electrons
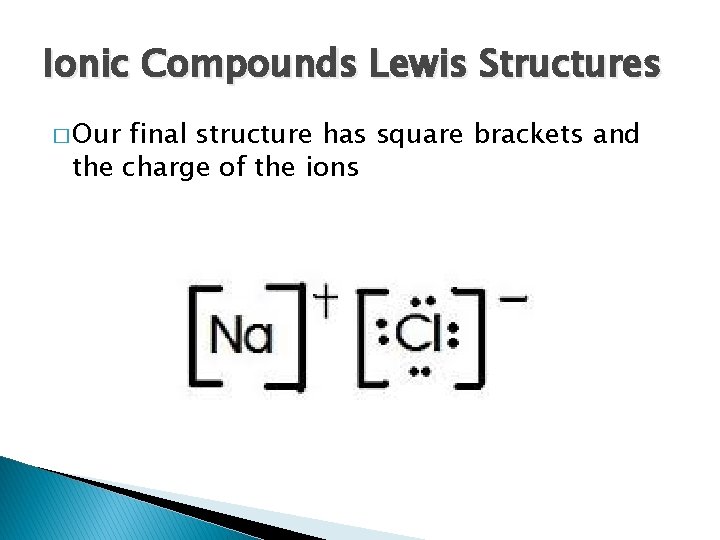
Ionic Compounds Lewis Structures � Our final structure has square brackets and the charge of the ions
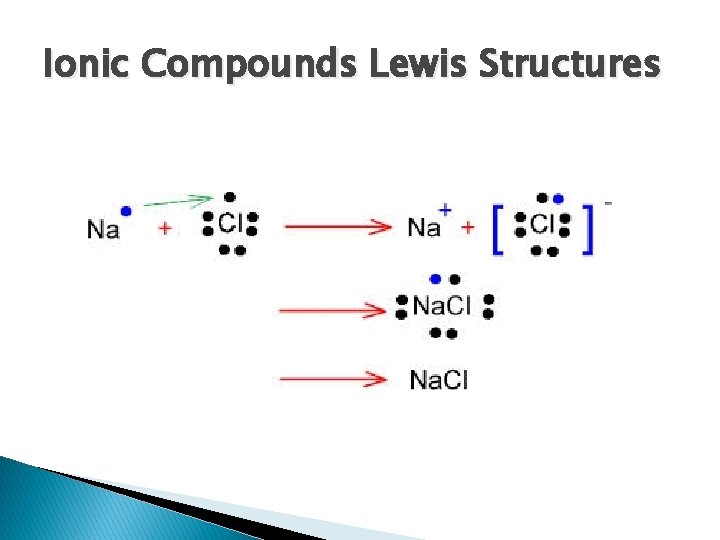
Ionic Compounds Lewis Structures
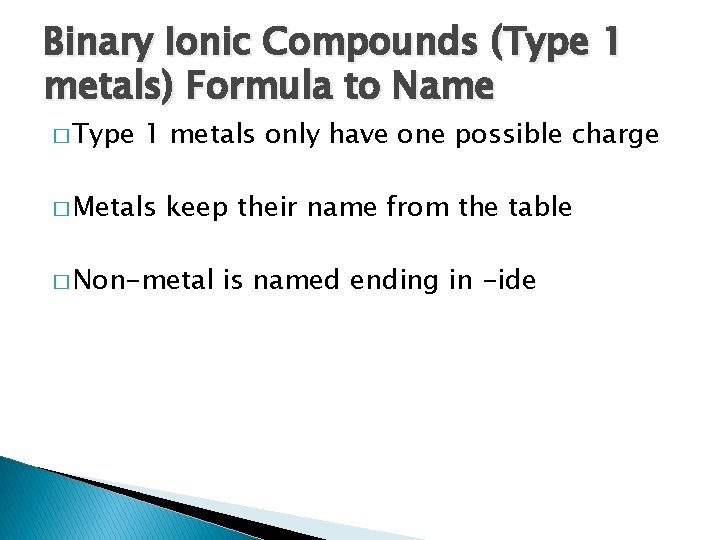
Binary Ionic Compounds (Type 1 metals) Formula to Name � Type 1 metals only have one possible charge � Metals keep their name from the table � Non-metal is named ending in -ide

Binary Ionic Compounds (Type 1 metals) Formula to Name � Mg. Cl 2 ◦ Magnesium chloride � Na. Cl ◦ Sodium chloride � Ag. Br ◦ Silver bromide
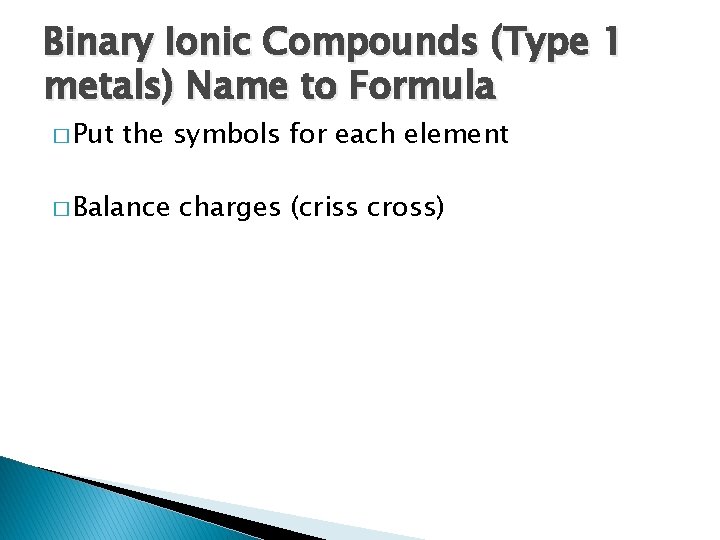
Binary Ionic Compounds (Type 1 metals) Name to Formula � Put the symbols for each element � Balance charges (criss cross)

Binary Ionic Compounds (Type 1 metals) Name to Formula � Cesium ◦ Cs. Br bromide � Cadmium ◦ Cd. F 2 fluoride � Aluminum ◦ Al 2 S 3 � Zinc ◦ Zn. S sulfide

Roman Numerals � I=1 � II=2 � III=3 � IV=4 � V=5 � VI=6 � VII=7 � VIII=8 � IX=9 � X=10
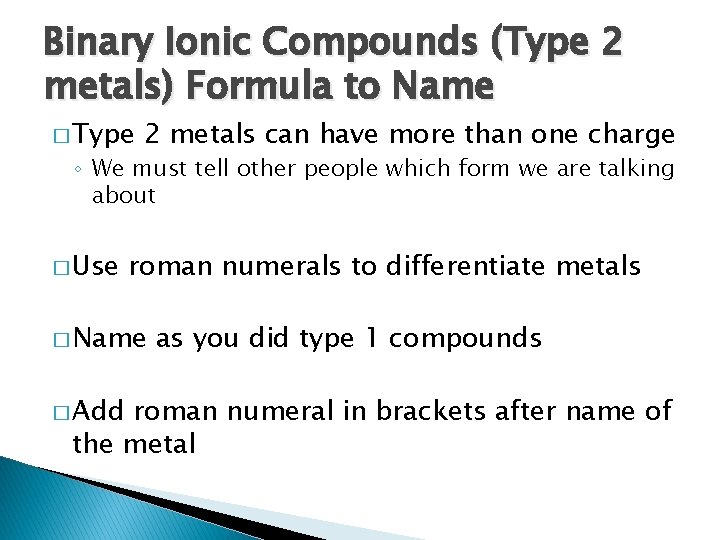
Binary Ionic Compounds (Type 2 metals) Formula to Name � Type 2 metals can have more than one charge ◦ We must tell other people which form we are talking about � Use roman numerals to differentiate metals � Name � Add as you did type 1 compounds roman numeral in brackets after name of the metal

Binary Ionic Compounds (Type 2 metals) Formula to Name � Au. Cl 3 ◦ Gold (III) chloride � Nb. N ◦ Niobium (I) nitride � VBr 5 ◦ Vanadium (V) bromide
Binary Ionic Compounds (Type 2 metals) Name to Formula � Important to remember the roman numeral tells you CHARGE not how many atoms � Write � Put symbols for the metal and non-metal roman numeral as charge � Balance charges (criss cross)

Binary Ionic Compounds (Type 2 metals) Name to Formula � Iron (II) bromide ◦ Fe. Br 2 � Nickel ◦ Cu. N � Lead (III) nitride (IV) oxide ◦ Pb. O 2

Polyatomic Ions � In your booklet � Charged chemical species composed of two or more atoms � Act as a unit
Polyatomic Ions Name to Formula � Follow the rules for the type of compound you are using � If there are multiples of the polytomic ion, use brackets ◦ Remember they act as a unit
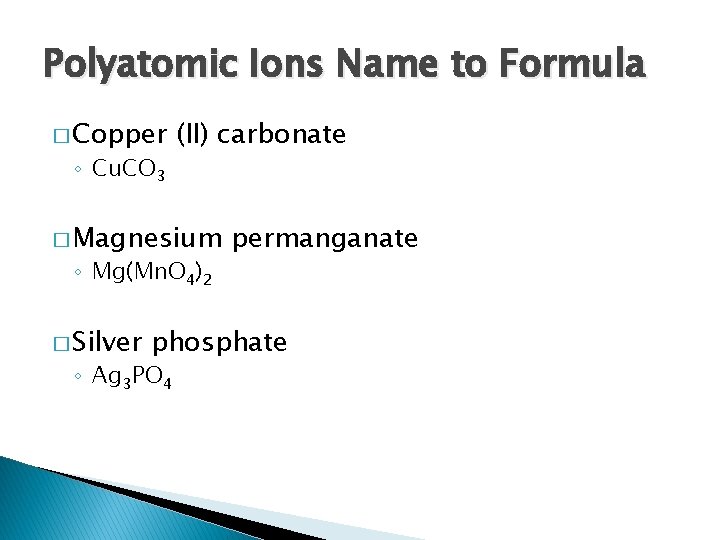
Polyatomic Ions Name to Formula � Copper ◦ Cu. CO 3 (II) carbonate � Magnesium ◦ Mg(Mn. O 4)2 � Silver permanganate phosphate ◦ Ag 3 PO 4
Polyatomic Ions Formula to Name � Name by following the rules for the type of compound you are using � Don’t change the name of the polyatomic ion
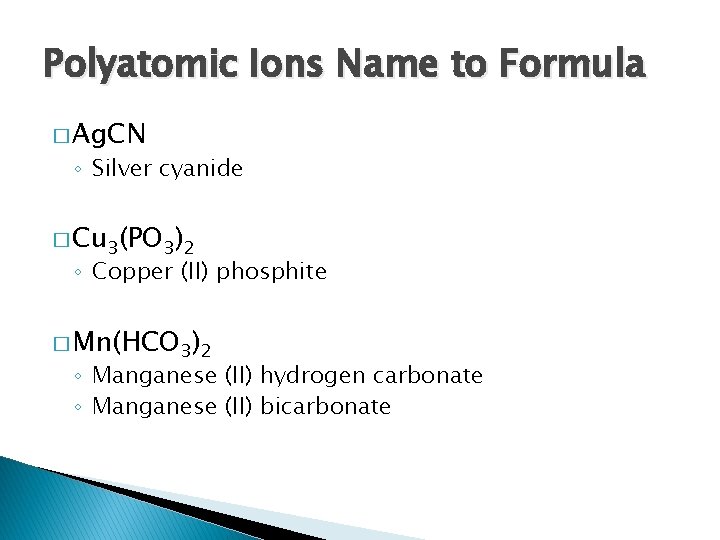
Polyatomic Ions Name to Formula � Ag. CN ◦ Silver cyanide � Cu 3(PO 3)2 ◦ Copper (II) phosphite � Mn(HCO 3)2 ◦ Manganese (II) hydrogen carbonate ◦ Manganese (II) bicarbonate
Hydrates � Formed by the addition of water or its components to another substance � Substances anhydrous � Water without water are called molecules form lattice around central compound

Prefixes � Mono=1 � Di=2 � Tri=3 � Tetra=4 � Penta=5 � Hexa=6 � Hepta=7 � Octa=8 � Nona=9 � Deca=10 � Tell you the number of water molecules that are present � Example: ◦ Hexahydrate = 6 water molecules

Hydrates Formula to Name � Add the base compound by following rules “hydrate” with the appropriate prefix

Hydrates Formula to Name � Li. Cl. O 4 • 3 H 2 O ◦ Lithium perchlorate trihydrate � Ni. SO 4 • 6 H 2 O ◦ Nickel (II) sulfate hexahydrate

Hydrates Name to Formula � Write the formula for the base compound by following previous rules � Separate water molecules from central compound with “ • ” � Write H 2 O with appropriate coefficient

Hydrates Name to Formula � Copper (II) sulfate pentahydrate ◦ Cu. SO 4 • 5 H 2 O � Magnesium carbonate pentahydrate ◦ Mg. CO 3 • 5 H 2 O
Covalent Bonds � Bonds made between non-metal atoms � Electron sharing due to similar affinities for electrons � No transfer of electrons � Smallest unit is the molecule ◦ 1 C and 4 H bonded together is one molecule
Covalent Compounds � Low melting and boiling points � Pliable � Do in solid form not conduct electricity when dissolved ◦ Do not ionize in solution
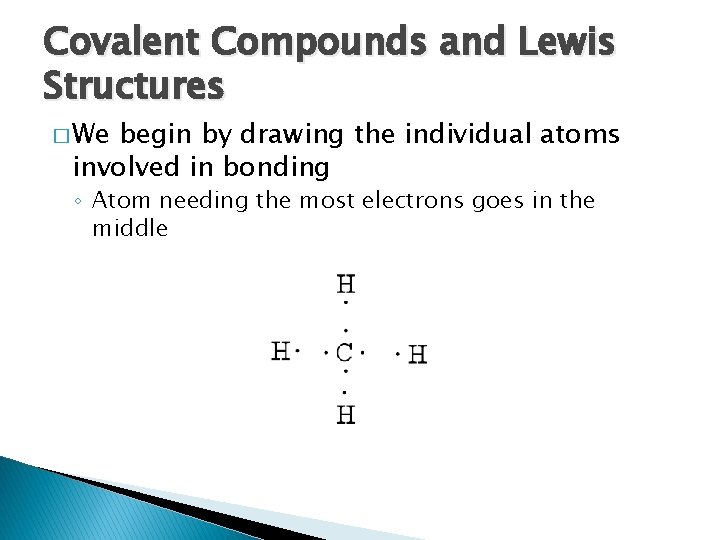
Covalent Compounds and Lewis Structures � We begin by drawing the individual atoms involved in bonding ◦ Atom needing the most electrons goes in the middle
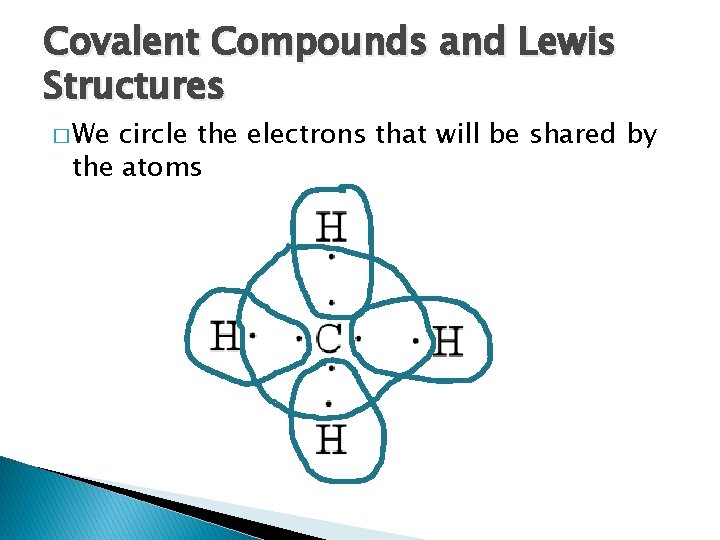
Covalent Compounds and Lewis Structures � We circle the electrons that will be shared by the atoms
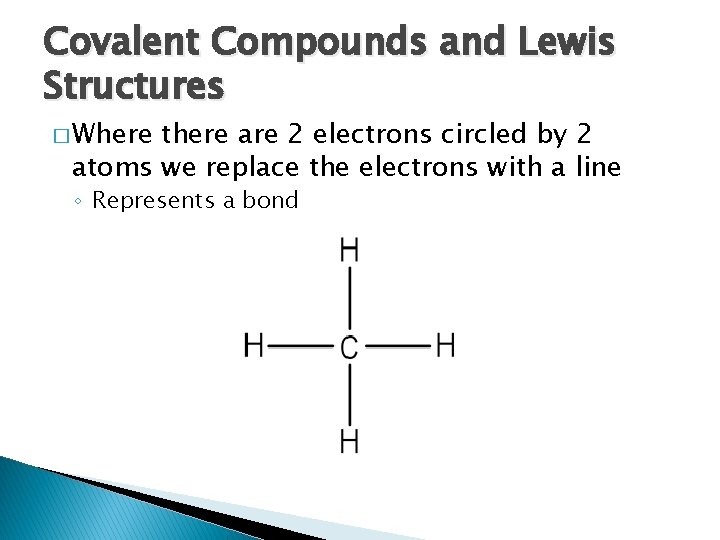
Covalent Compounds and Lewis Structures � Where there are 2 electrons circled by 2 atoms we replace the electrons with a line ◦ Represents a bond
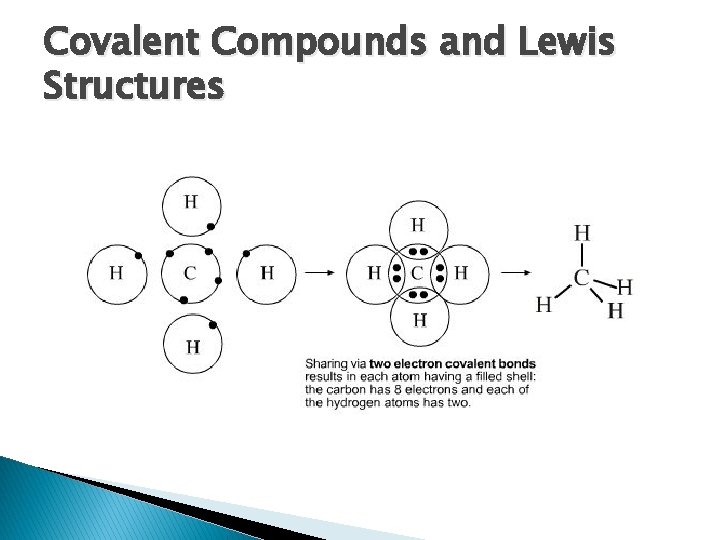
Covalent Compounds and Lewis Structures
Covalent Compounds and Lewis Structures � When 2 electrons are shared it is a single bond (1 shared pair) � Can have multiple bonds � 4 electrons shared (2 shared pairs) = double bond � 6 electrons shared (3 shared pairs)= triple bond
Covalent Compounds and Lewis Structures � For more complicated compounds: ◦ Add up total valence electrons of bonding atoms �This is the number of electrons we need in our final structure ◦ Draw one bond between the central atom and the other bonding atoms �Each bond counts as using up 2 of the electrons we started with ◦ Draw in the valence electrons on the atoms ◦ Borrow electrons to give the central atom a full octet
Covalent Compounds and Lewis Structures � Draw � CO 2 � HCN � COS the Lewis Structure of the following:

Covalent Compounds Formula to Name � Prefixes � Mono=1 � Di=2 � Tri=3 � Tetra=4 � Penta=5 � Hexa=6 � Hepta=7 � Octa=8 � Nona=9 � Deca=10
Covalent Compounds Formula to Name � Write the names of the elements present � Change � Add ending to –ide for last element prefixes to match the number of atoms of each element
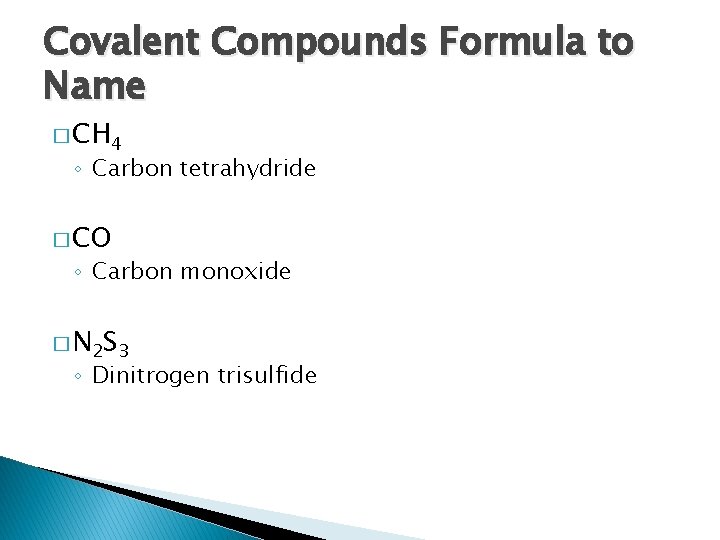
Covalent Compounds Formula to Name � CH 4 ◦ Carbon tetrahydride � CO ◦ Carbon monoxide � N 2 S 3 ◦ Dinitrogen trisulfide
Covalent Compounds Name to Formula � Write the symbols for each element in the compound � Use the prefixes as the subscripts in the formula
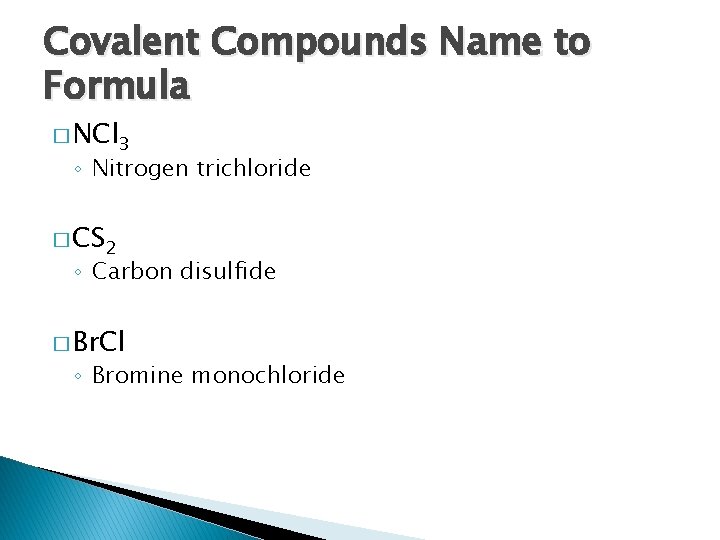
Covalent Compounds Name to Formula � NCl 3 ◦ Nitrogen trichloride � CS 2 ◦ Carbon disulfide � Br. Cl ◦ Bromine monochloride
- Slides: 62