Unit 3 Bonding Intermolecular Forces Polarity and Solubility
Unit 3: Bonding Intermolecular Forces

Polarity and Solubility l “like dissolves like” l How do we explain this? ? ?
Intermolecular Forces l Intermolecular forces: weak and mostly temporary attractions between molecules l Need to know: London dispersion forces (induced dipole) l Dipole-dipole forces l Hydrogen bonding forces l Ion-dipole forces l
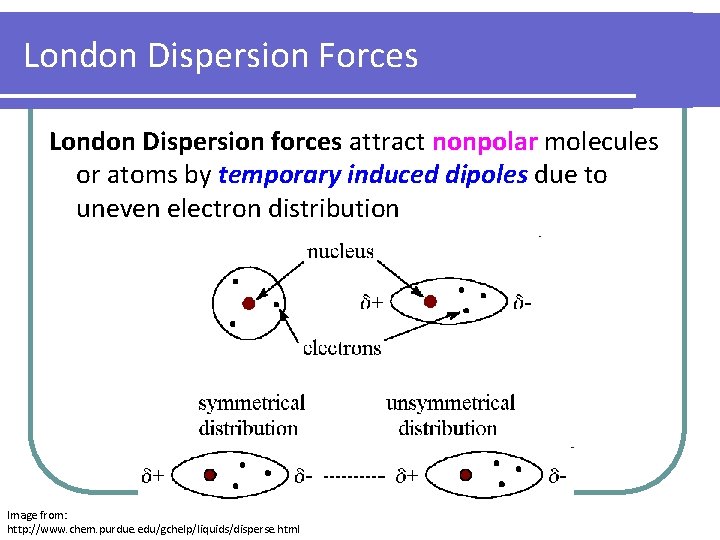
London Dispersion Forces London Dispersion forces attract nonpolar molecules or atoms by temporary induced dipoles due to uneven electron distribution Image from: http: //www. chem. purdue. edu/gchelp/liquids/disperse. html
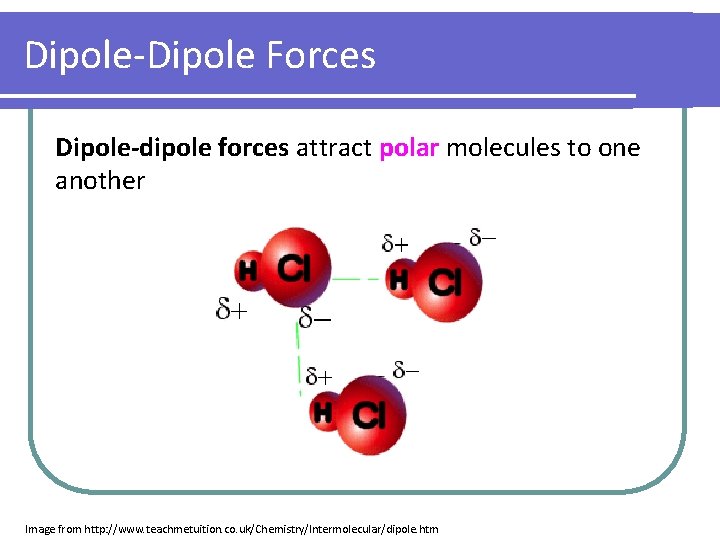
Dipole-Dipole Forces Dipole-dipole forces attract polar molecules to one another Image from http: //www. teachmetuition. co. uk/Chemistry/Intermolecular/dipole. htm
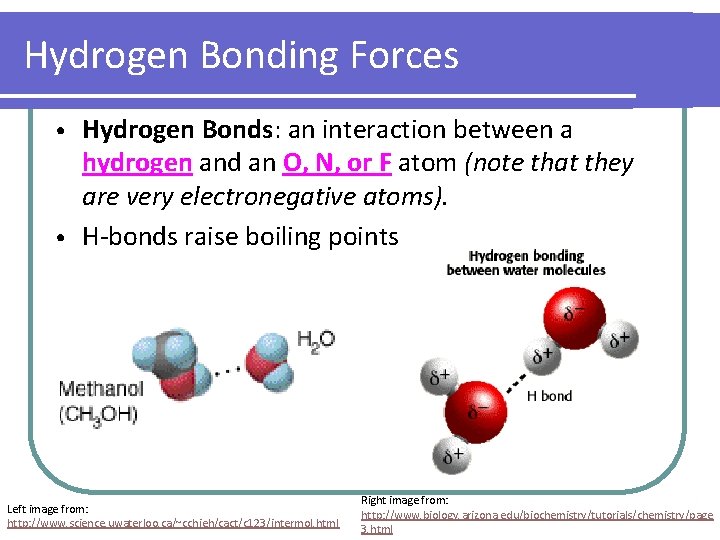
Hydrogen Bonding Forces Hydrogen Bonds: an interaction between a hydrogen and an O, N, or F atom (note that they are very electronegative atoms). • H-bonds raise boiling points • Left image from: http: //www. science. uwaterloo. ca/~cchieh/cact/c 123/intermol. html Right image from: http: //www. biology. arizona. edu/biochemistry/tutorials/chemistry/page 3. html
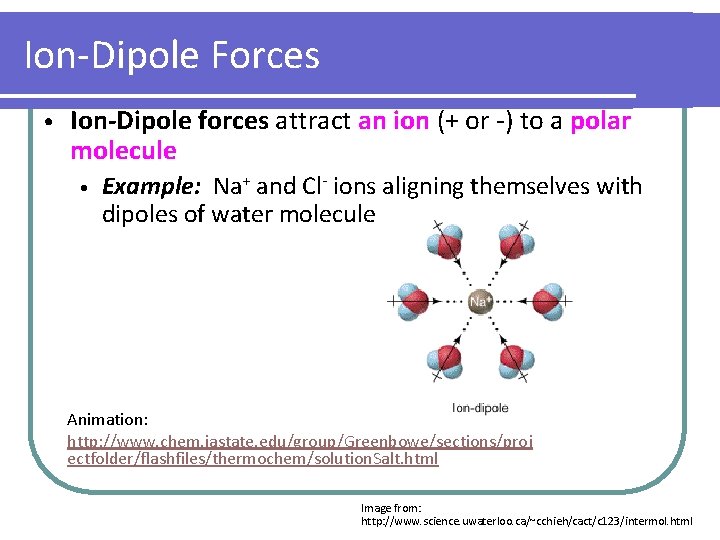
Ion-Dipole Forces • Ion-Dipole forces attract an ion (+ or -) to a polar molecule • Example: Na+ and Cl- ions aligning themselves with dipoles of water molecule Animation: http: //www. chem. iastate. edu/group/Greenbowe/sections/proj ectfolder/flashfiles/thermochem/solution. Salt. html Image from: http: //www. science. uwaterloo. ca/~cchieh/cact/c 123/intermol. html
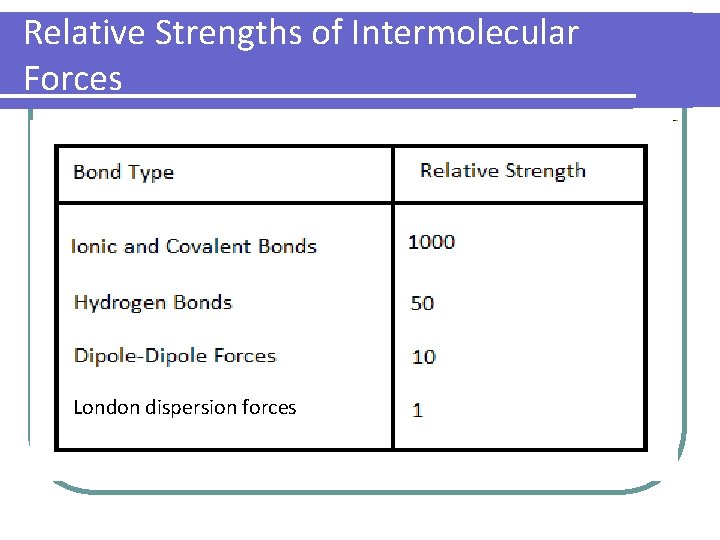
Relative Strengths of Intermolecular Forces London dispersion forces
Other Bond-Like Forces • A nice summary of intermolecular forces: http: //www. wisc-online. com/objects/index_tj. asp? obj. ID=GCH 6804
- Slides: 9