Unit 3 Biochemistry DAY 1 MATTER PROPERTIES OF

Unit 3 Biochemistry DAY 1: MATTER, PROPERTIES OF WATER, SOLUTIONS/PH, AND CHEMICAL REACTIONS/ENZYMES

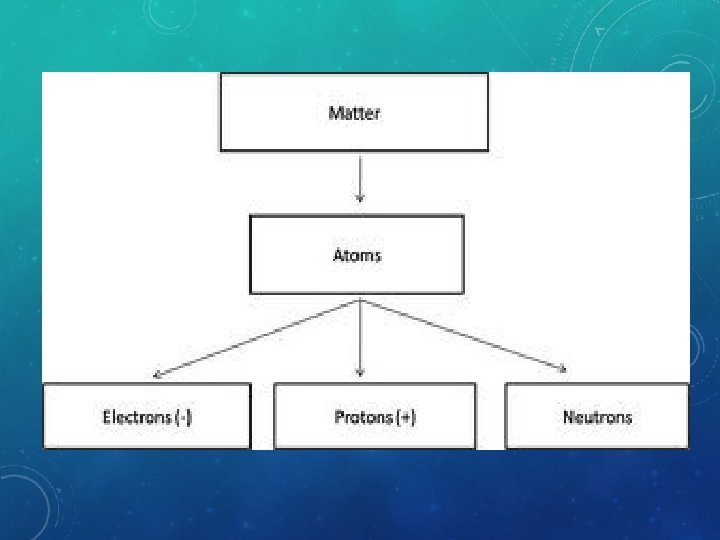
What is Matter? Anything that has mass and volume.

What is the smallest unit of matter? Hint: not a Chihuahua!
An Atom!
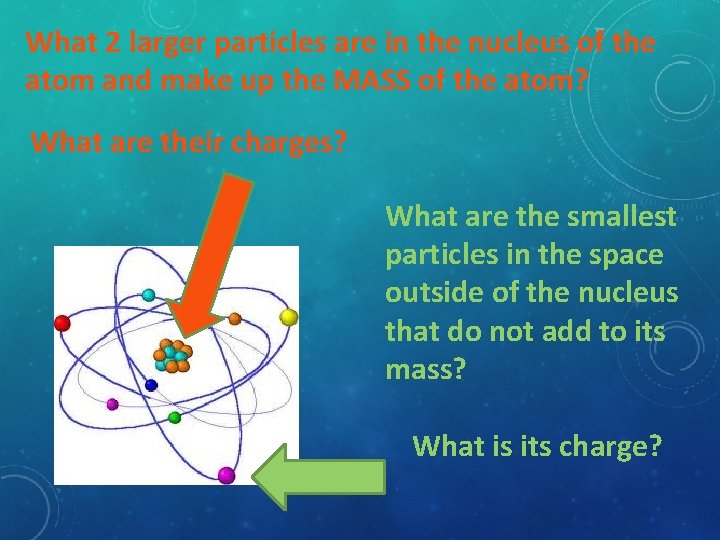
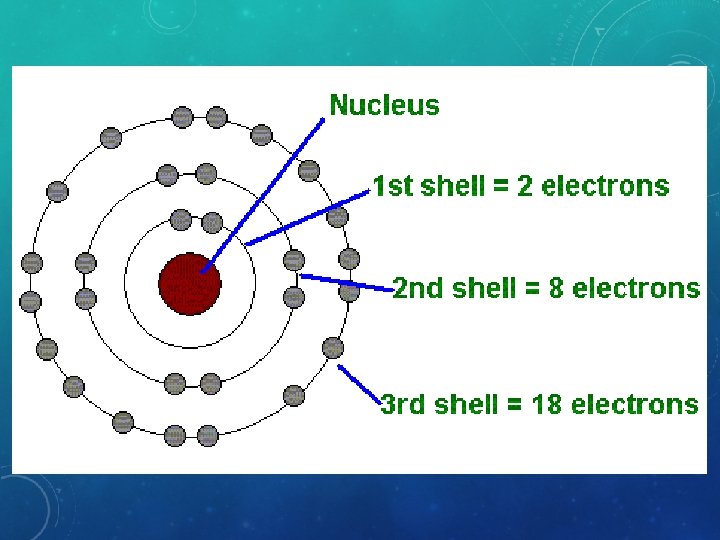
What 2 larger particles are in the nucleus of the atom and make up the MASS of the atom? What are their charges? What are the smallest particles in the space outside of the nucleus that do not add to its mass? What is its charge?
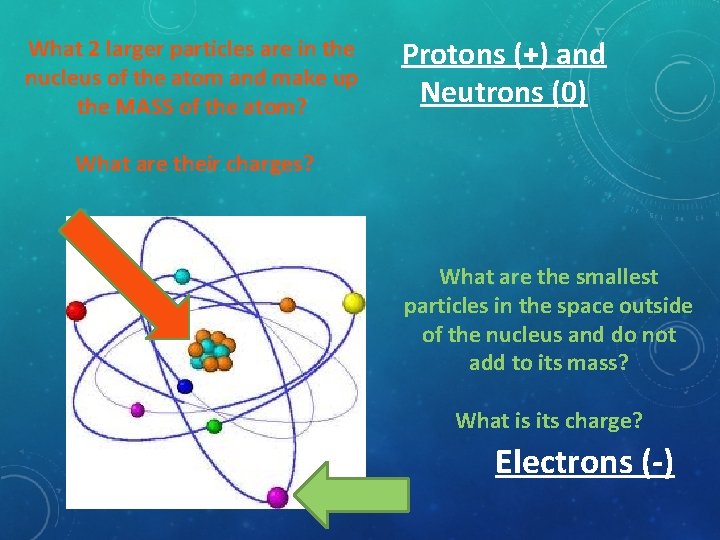
What 2 larger particles are in the nucleus of the atom and make up the MASS of the atom? Protons (+) and Neutrons (0) What are their charges? What are the smallest particles in the space outside of the nucleus and do not add to its mass? What is its charge? Electrons (-)

Electron Shell Level First Level Second Level Third Level Maximum Number of Electrons in each Shell ? ? ?


What is an Hint: It’s not the skateboarding company! ?

An element is “pure stuff”. It is only one kind of atom in matter such as pure gold (Au) from the periodic table. Other Examples: Carbon Hydrogen Oxygen Sodium Phosphorous
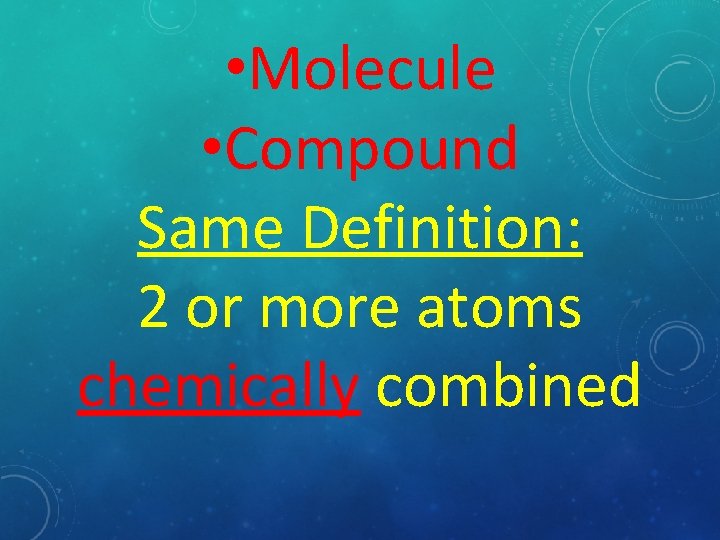
• Molecule • Compound Same Definition: 2 or more atoms chemically combined
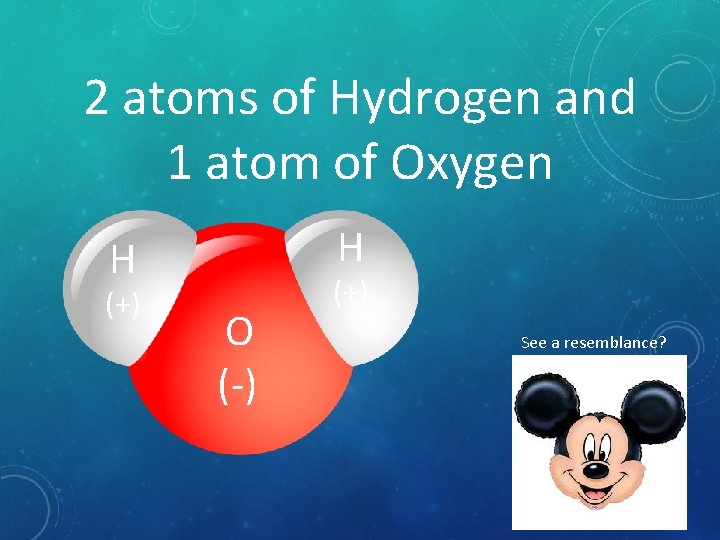
2 atoms of Hydrogen and 1 atom of Oxygen H H (+) O (-) (+) See a resemblance?
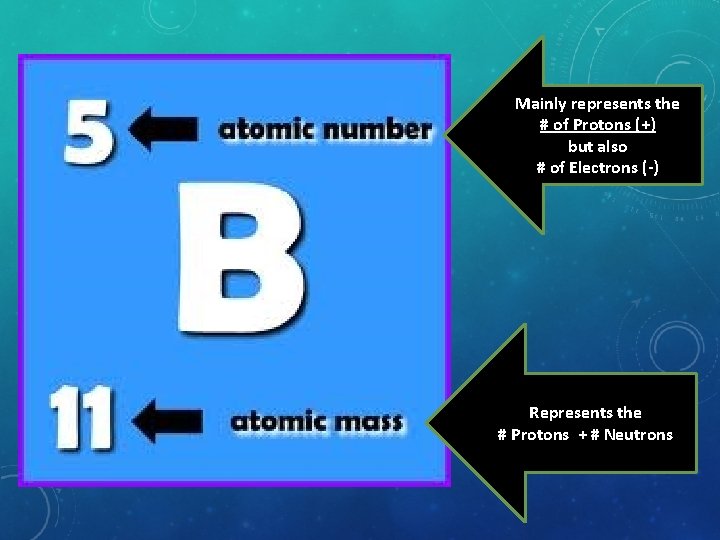
Mainly represents the # of Protons (+) but also # of Electrons (-) Represents the # Protons + # Neutrons

What are…. .
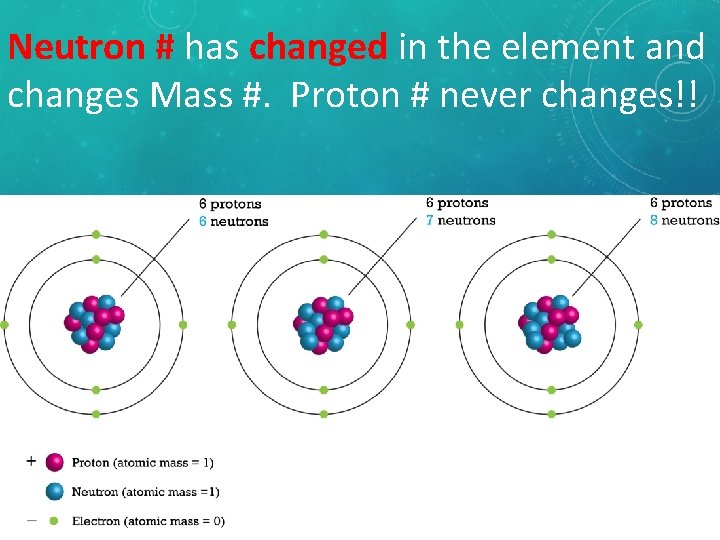
Neutron # has changed in the element and changes Mass #. Proton # never changes!! Isotopes of Carbon
ION
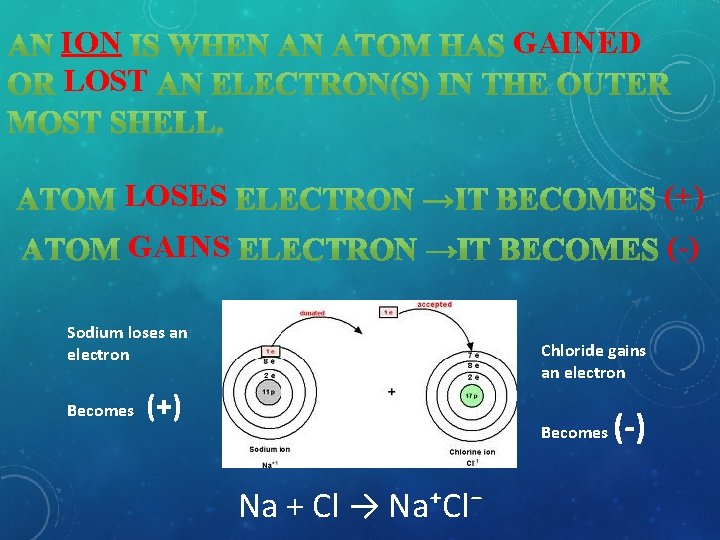
ION LOST GAINED LOSES (+) GAINS (-) Sodium loses an electron Becomes Chloride gains an electron (+) Becomes Na + Cl → Na⁺Cl⁻ (-)
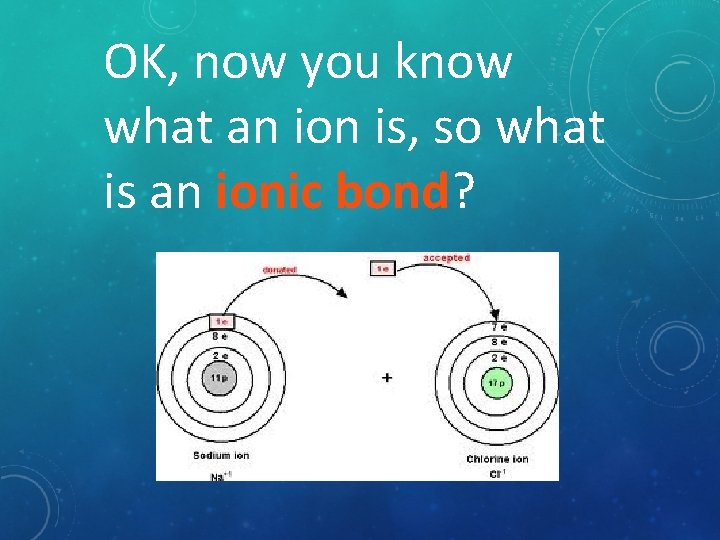
OK, now you know what an ion is, so what is an ionic bond?
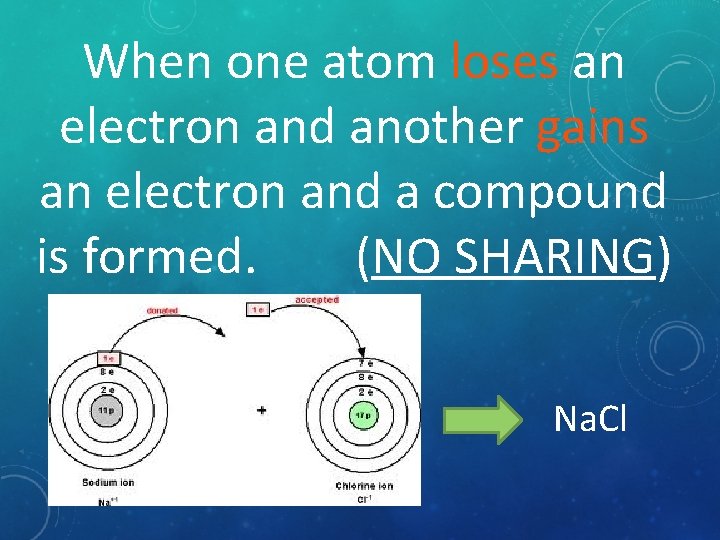
When one atom loses an electron and another gains an electron and a compound is formed. (NO SHARING) Na. Cl

What is a covalent bond? Think:
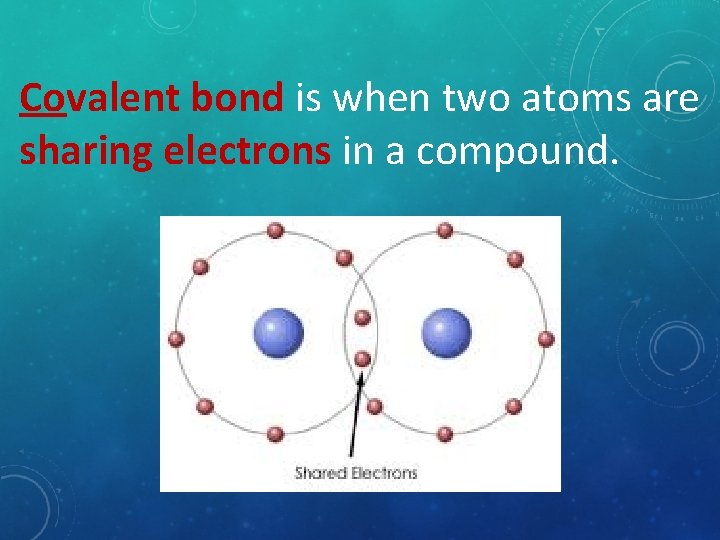
Covalent bond is when two atoms are sharing electrons in a compound.

OTHER TYPES OF BONDS • Hydrogen Bonds – bonds between (slightly positive charges of) hydrogen molecules and (slightly negative charges of) other molecules—more to come later! • Van der waals forces – weakest of bonds. Example: bond between a gecko’s foot and molecules on the wall

INTERMISSION: PROPERTIES OF WATER
Chemical Reactions What are the reactants and products of this reaction? A+B→C
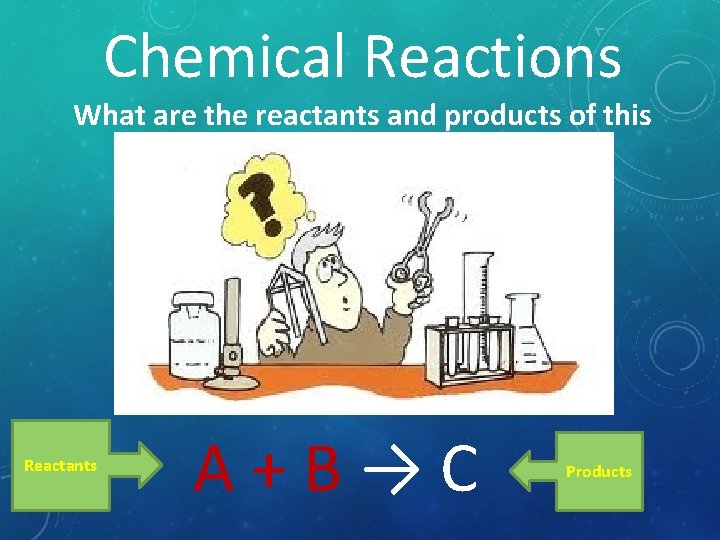
Chemical Reactions What are the reactants and products of this reaction? Reactants A+B→C Products
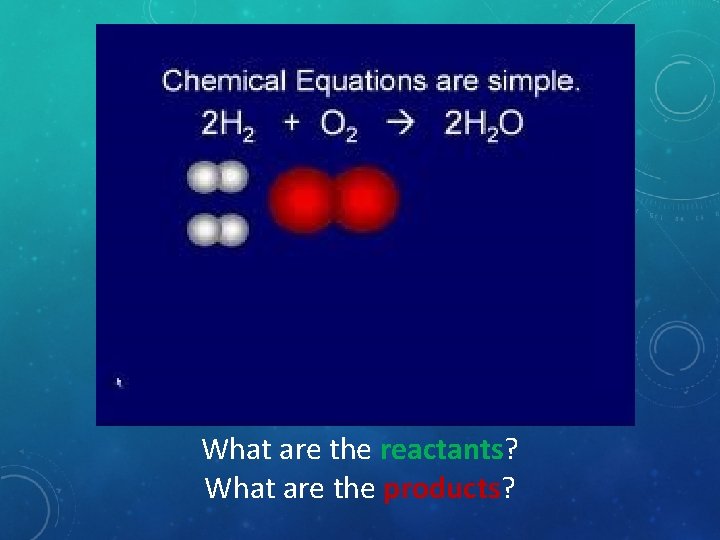
What are the reactants? What are the products?
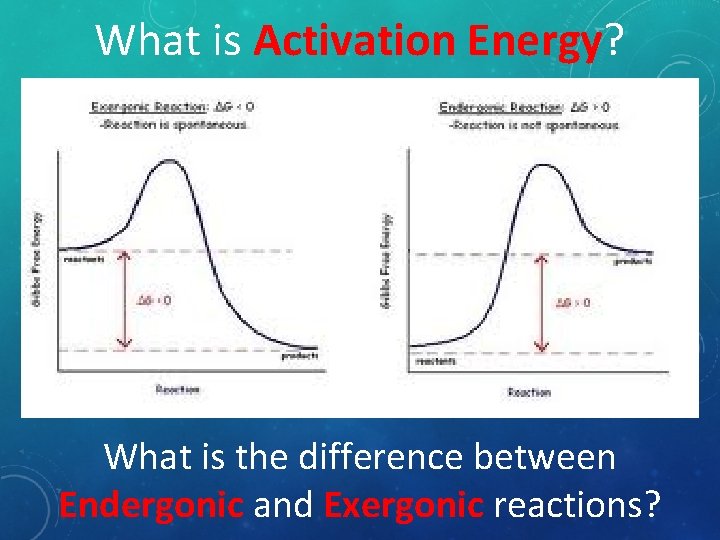
What is Activation Energy? What is the difference between Endergonic and Exergonic reactions?
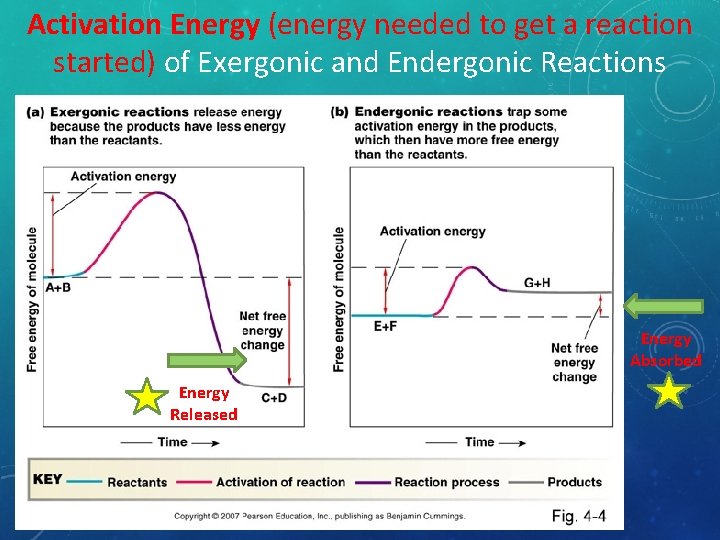
Activation Energy (energy needed to get a reaction started) of Exergonic and Endergonic Reactions Energy Absorbed Energy Released
Energy released during a chemical reaction is in the form of……… Heat, Light, and or Sound
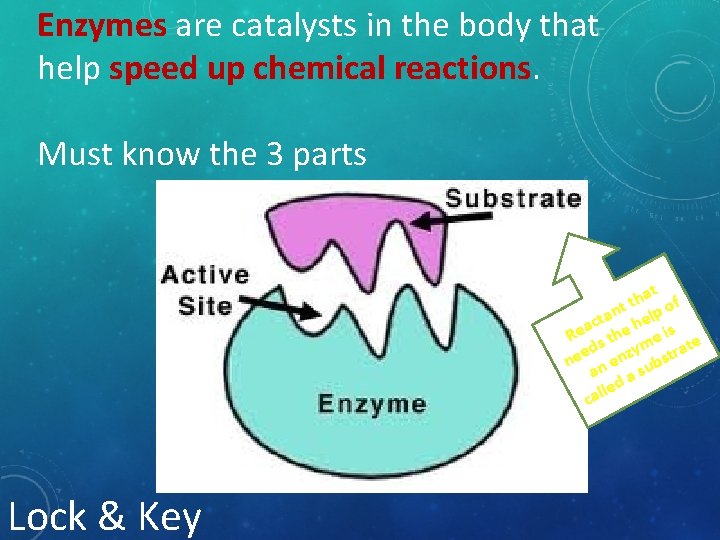
Enzymes are catalysts in the body that help speed up chemical reactions. Must know the 3 parts at f h t nt elp o a t h ac Re s the e is te d ym stra e z e n n n e ub a as d le cal Lock & Key

ENZYMES ARE AFFECTED BY… • p. H • Temperature • Amount of substrate • Amount of enzyme • Presence of activators or inhibitors
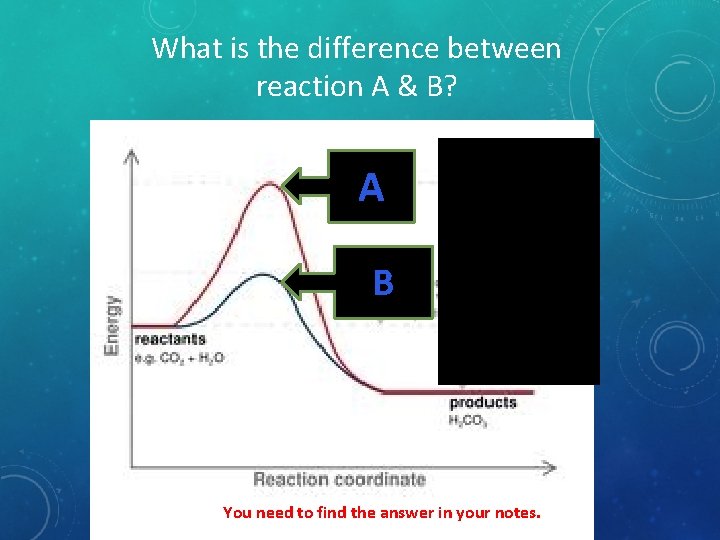
What is the difference between reaction A & B? A B You need to find the answer in your notes.
- Slides: 34