Unit 3 Balancing Equations Types of Reactions Chapter

Unit 3: Balancing Equations, Types of Reactions Chapter 8
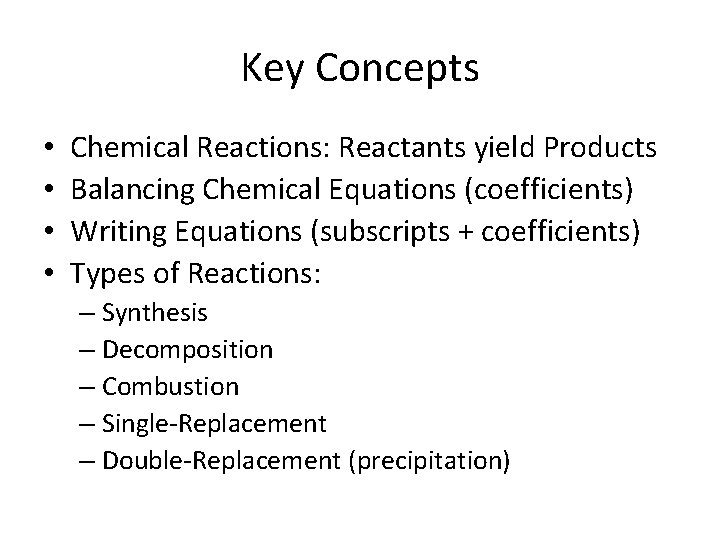
Key Concepts • • Chemical Reactions: Reactants yield Products Balancing Chemical Equations (coefficients) Writing Equations (subscripts + coefficients) Types of Reactions: – Synthesis – Decomposition – Combustion – Single-Replacement – Double-Replacement (precipitation)

Chemical Reactions • Chemical Reaction: substances change into new substances with different physical and chemical properties – Reactants: go into a chemical reaction – Products: come out of a chemical reaction
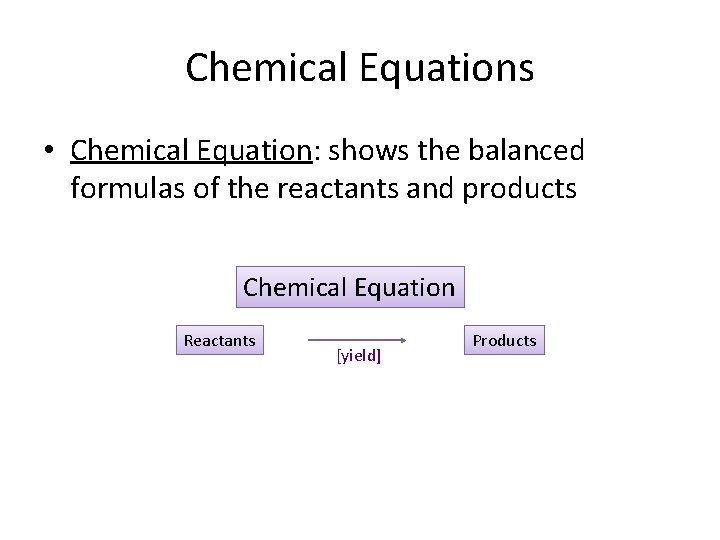
Chemical Equations • Chemical Equation: shows the balanced formulas of the reactants and products Chemical Equation Reactants [yield] Products
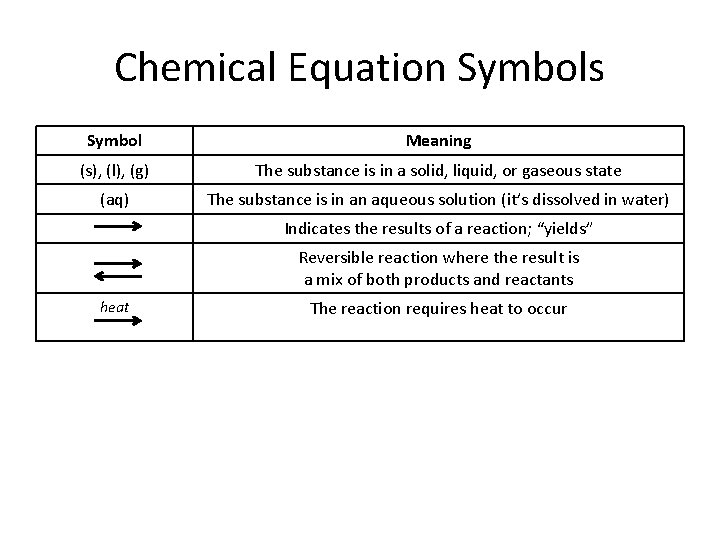
Chemical Equation Symbols Symbol Meaning (s), (l), (g) The substance is in a solid, liquid, or gaseous state (aq) The substance is in an aqueous solution (it’s dissolved in water) Indicates the results of a reaction; “yields” Reversible reaction where the result is a mix of both products and reactants heat The reaction requires heat to occur
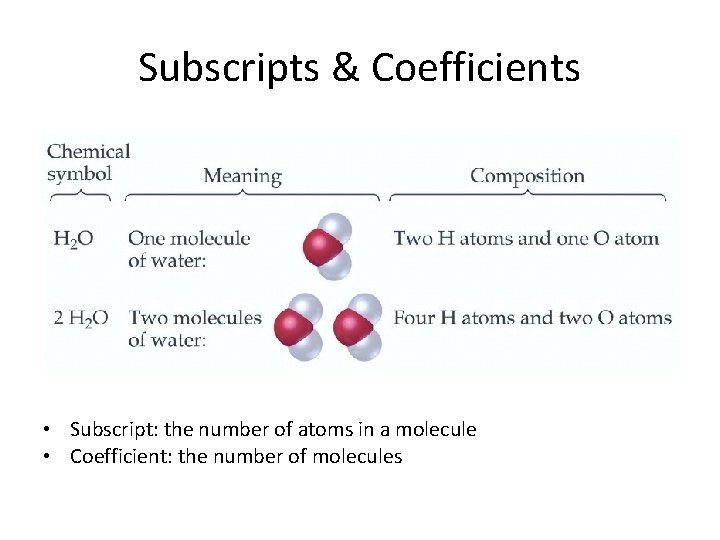
Subscripts & Coefficients • Subscript: the number of atoms in a molecule • Coefficient: the number of molecules
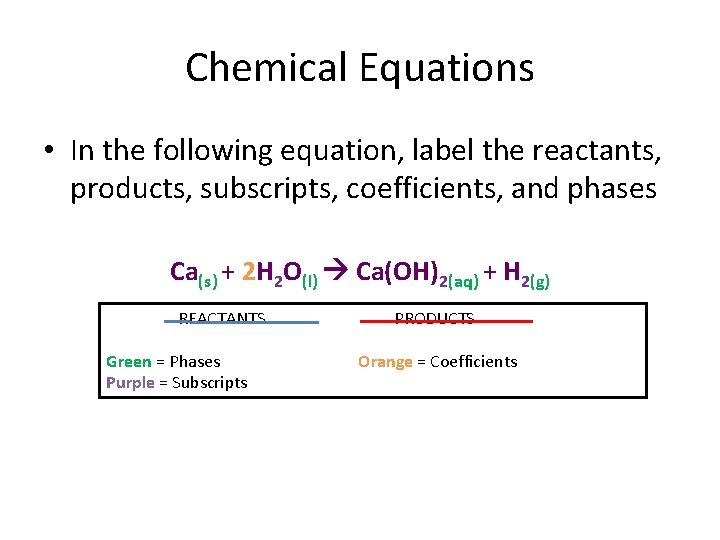
Chemical Equations • In the following equation, label the reactants, products, subscripts, coefficients, and phases Ca(s) + 2 H 2 O(l) Ca(OH)2(aq) + H 2(g) REACTANTS Green = Phases Purple = Subscripts PRODUCTS Orange = Coefficients
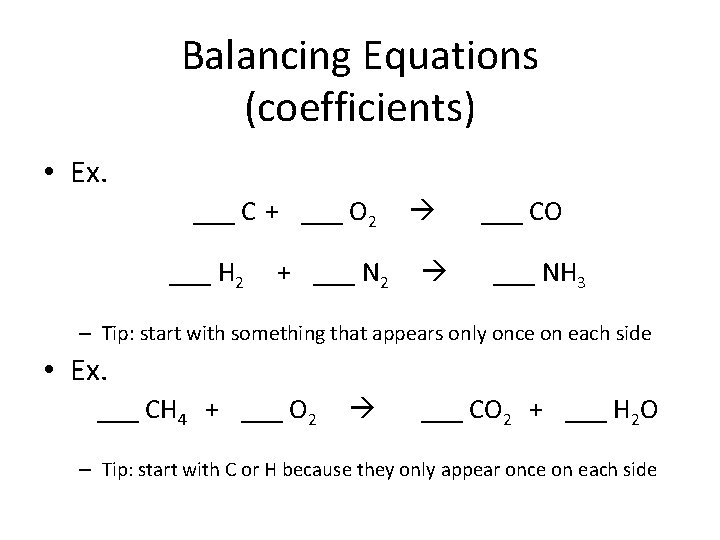
Balancing Equations (coefficients) • Ex. ___ C + ___ O 2 ___ H 2 + ___ N 2 ___ CO ___ NH 3 – Tip: start with something that appears only once on each side • Ex. ___ CH 4 + ___ O 2 ___ CO 2 + ___ H 2 O – Tip: start with C or H because they only appear once on each side
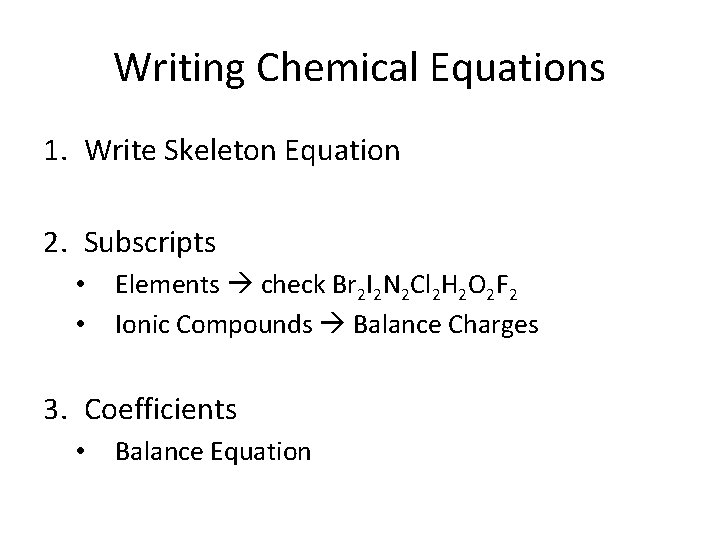
Writing Chemical Equations 1. Write Skeleton Equation 2. Subscripts • • Elements check Br 2 I 2 N 2 Cl 2 H 2 O 2 F 2 Ionic Compounds Balance Charges 3. Coefficients • Balance Equation

Writing Equations (subscripts + coefficients) • Ex. hydrogen was combined with oxygen to yield water • Ex. nitrogen and hydrogen yield ammonia (NH 3) • Ex. aluminum bromide plus chlorine yield aluminum chloride plus bromine
Types of Reactions • • • Synthesis Reactions Decomposition Reactions Combustion Reactions Single-Replacement Reactions Double-Replacement (precipitation) Reactions
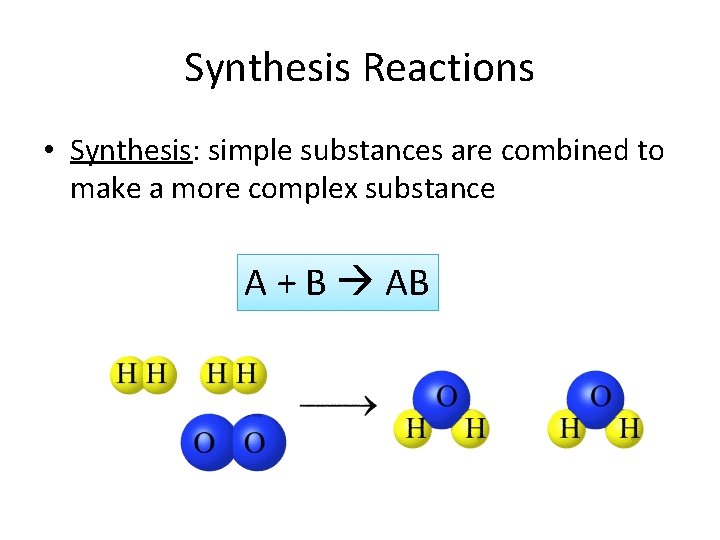
Synthesis Reactions • Synthesis: simple substances are combined to make a more complex substance A + B AB
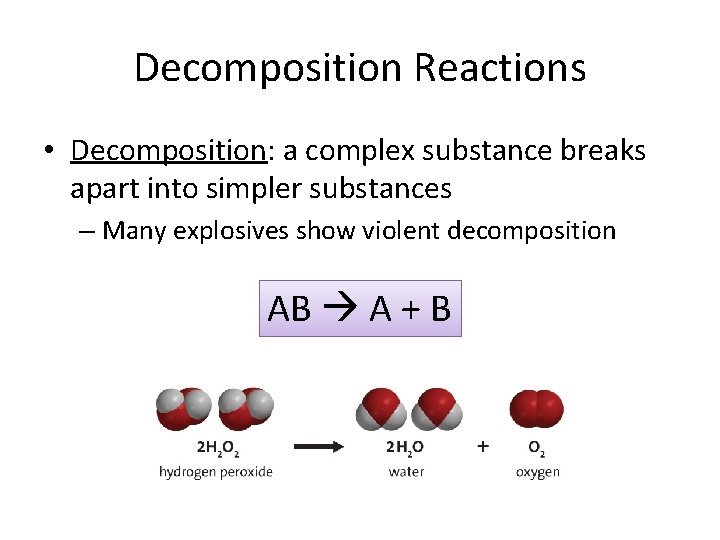
Decomposition Reactions • Decomposition: a complex substance breaks apart into simpler substances – Many explosives show violent decomposition AB A + B
Combustion Reactions • Combustion: a substance burns in oxygen to form oxides A + O 2 A XO Y • Combustion with a hydrocarbon will always produce CO 2 + H 2 O CXHY + O 2 CO 2 + H 2 O
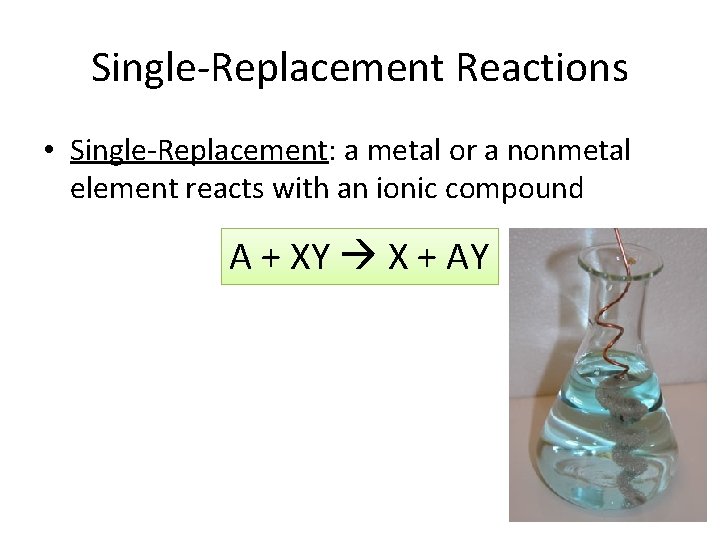
Single-Replacement Reactions • Single-Replacement: a metal or a nonmetal element reacts with an ionic compound A + XY X + AY
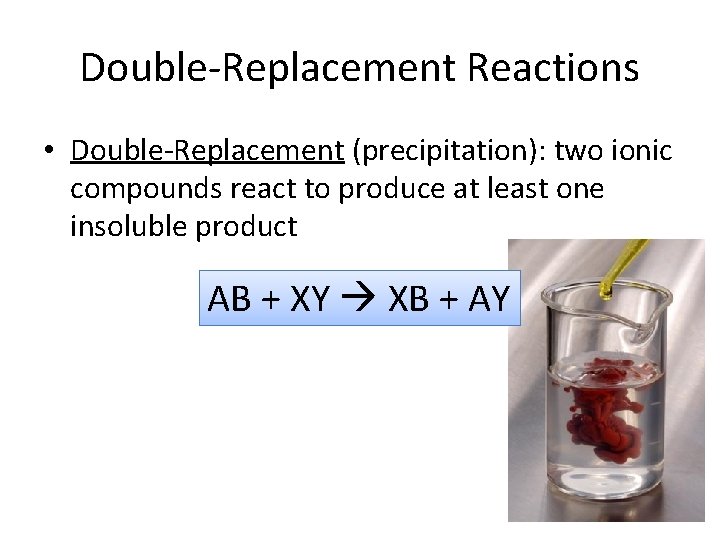
Double-Replacement Reactions • Double-Replacement (precipitation): two ionic compounds react to produce at least one insoluble product AB + XY XB + AY
- Slides: 16