Unit 3 Balancing Equations Section 2 Balancing Techniques

Unit 3: Balancing Equations Section 2: Balancing Techniques

Counting Atoms • To balance equations, you must be able to count the atoms given *The following affects the number of atoms in a substance – Subscripts – Parentheses – Coefficients

Subscripts • Small-sized numbers that are written after the element symbol and placed slightly lower – H 2 O • 2 - H atoms • 1 - O atom – NH 4 NO 3 • 2 - N atoms • 4 - H atoms • 3 - O atoms
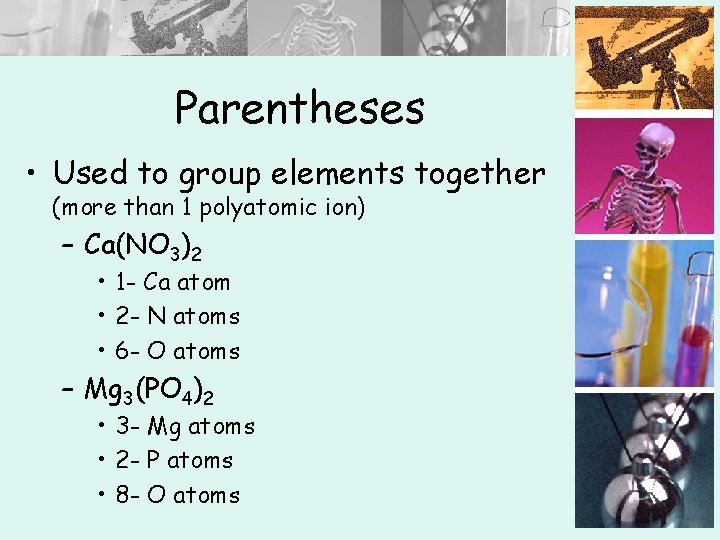
Parentheses • Used to group elements together (more than 1 polyatomic ion) – Ca(NO 3)2 • 1 - Ca atom • 2 - N atoms • 6 - O atoms – Mg 3(PO 4)2 • 3 - Mg atoms • 2 - P atoms • 8 - O atoms
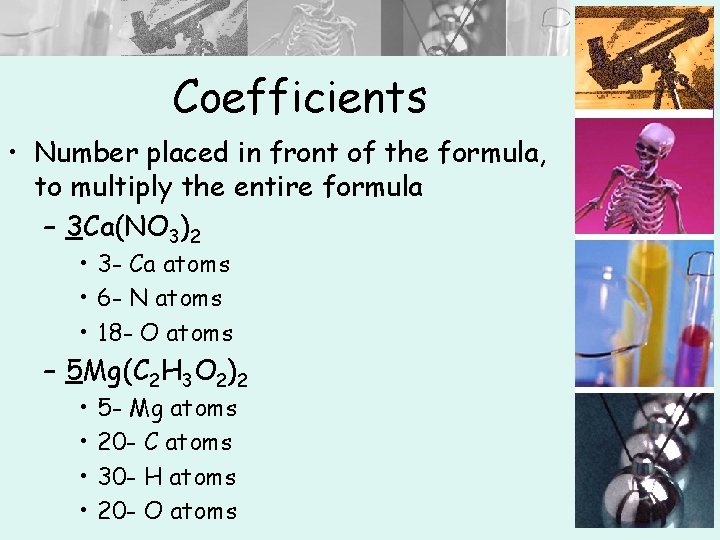
Coefficients • Number placed in front of the formula, to multiply the entire formula – 3 Ca(NO 3)2 • 3 - Ca atoms • 6 - N atoms • 18 - O atoms – 5 Mg(C 2 H 3 O 2)2 • • 5 - Mg atoms 20 - C atoms 30 - H atoms 20 - O atoms

Practice Time Answer questions 1 -6!

Balanced Equation • A chemist’s recipe – Tells what reactants and its amount to mix together – Tells what the end product(s) and their amount(s) will be

Balancing Rules • Can only add coefficients to balance, not subscripts • Start by counting up the total number of each element in the reactants and products • Never try to balance the number of oxygen (and usually hydrogen too) atoms first, start with another element • Try to start with an element that appears in only 1 substance on each side • Coefficients must be whole numbers
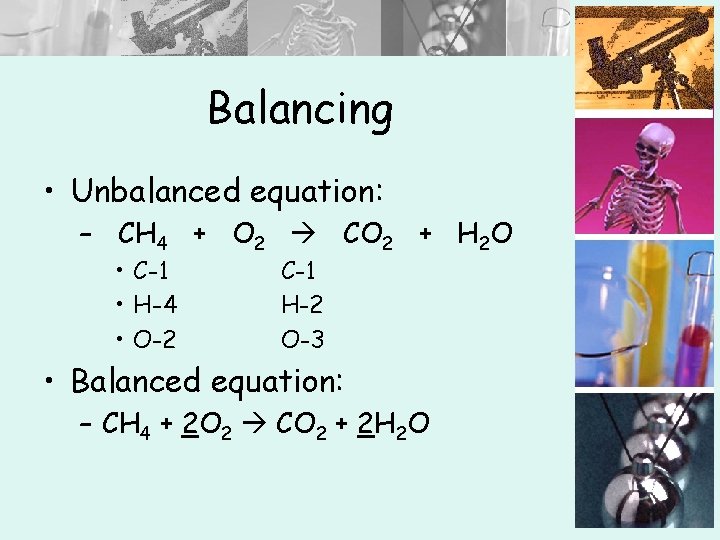
Balancing • Unbalanced equation: – CH 4 + O 2 CO 2 + H 2 O • C-1 • H-4 • O-2 C-1 H-2 O-3 • Balanced equation: – CH 4 + 2 O 2 CO 2 + 2 H 2 O
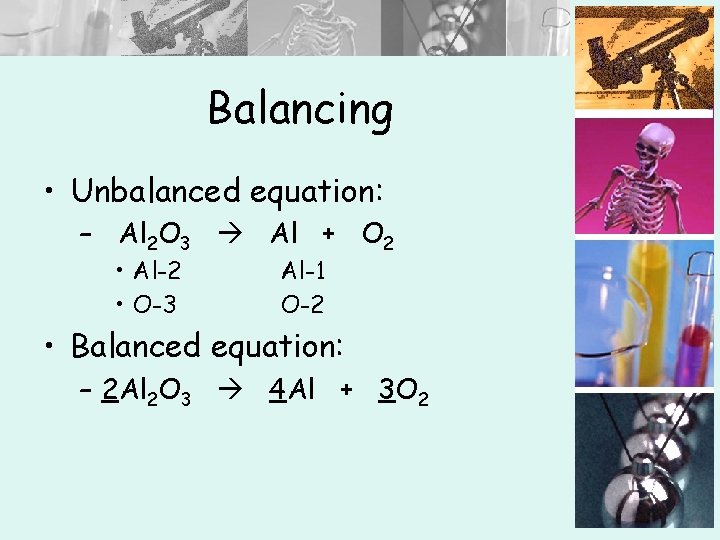
Balancing • Unbalanced equation: – Al 2 O 3 Al + O 2 • Al-2 • O-3 Al-1 O-2 • Balanced equation: – 2 Al 2 O 3 4 Al + 3 O 2

Practice Time Answer the questions!
- Slides: 11