Unit 3 Balancing Chemical Equations Section 3 Types
Unit 3: Balancing Chemical Equations Section 3: Types of Chemical Reactions
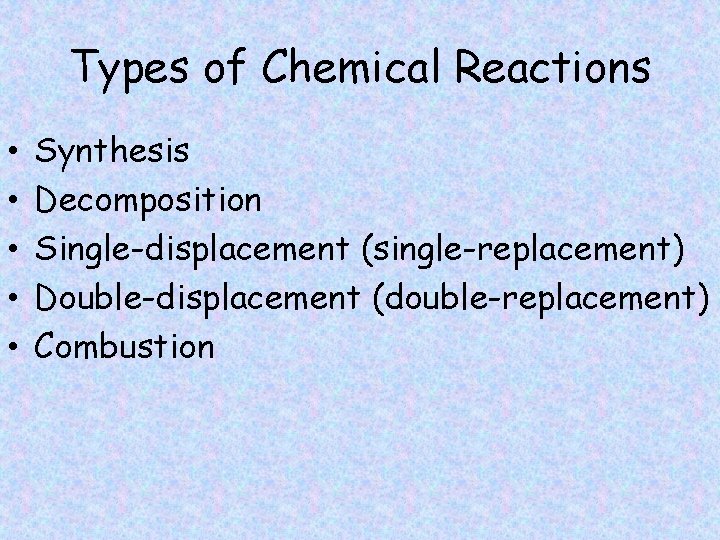
Types of Chemical Reactions • • • Synthesis Decomposition Single-displacement (single-replacement) Double-displacement (double-replacement) Combustion

Synthesis • Two substances combine to form a compound –A + X AX “ 2 things become 1” • Ex: 2 Mg + O 2 2 Mg. O

Decomposition • One compound breaks down into two different substances –AX A + X “ 1 thing breaks down into 2” • Ex: 2 Na. Cl 2 Na + Cl 2

Single-Displacement • An element replaces a similarly charged element in a compound – A + BX AX + B • Ex: 2 Na + Mg. Cl 2 Mg + 2 Na. Cl • “positive replaces positive” – Y + BX BY + X • Ex: Cl 2 + 2 KBr Br 2 + 2 KCl • “negative replaces negative”

Double-Displacement • Two original compounds form two new compounds –AX + BY AY + BX • Ex: Fe. S + 2 HCl Fe. Cl 2 + H 2 S

Combustion • A substance that combines with oxygen to form carbon dioxide and water –__? __ + O 2 CO 2 + H 2 O • Ex: C 6 H 12 O 6 + O 2 CO 2 + H 2 O

Practice Problems 1. 2. 3. 4. 5. 6. 7. N 2 + 3 H 2 2 NH 3 2 KCl. O 3 2 KCl + 3 O 2 2 H 2 + O 2 2 H 2 O C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O Fe. Cl 3 + 3 Na. OH Fe(OH)3 + 3 Na. Cl 2 K + Mg. Br 2 2 KBr + Mg 2 Ag. NO 3 + Mg. Cl 2 2 Ag. Cl + Mg(NO 3)2
- Slides: 8