Unit 3 Atomic Structure 3 2 Atomic Number

Unit 3: Atomic Structure 3. 2 Atomic Number, Mass Number, and Isotopes

After today, you will be able to… • Find the atomic mass, atomic number, and symbols of elements off of the Periodic Table. • Calculate the number of neutrons in a particular atom. • Explain what an isotope is.

Atomic Number • The number of protons in an element. • It is always a whole number. • Example: Oxygen’s atomic number is 8, so there are 8 protons in Oxygen’s nucleus.
Atomic Number • For neutral elements (no charge) the # of p+ = # of e • The number of protons will never change for an element. • It is like our social security number, it identifies them.

Atomic Mass • Read right off the Periodic Table. • The number will have a decimal. • It is the weighted average of all the elements isotopes.
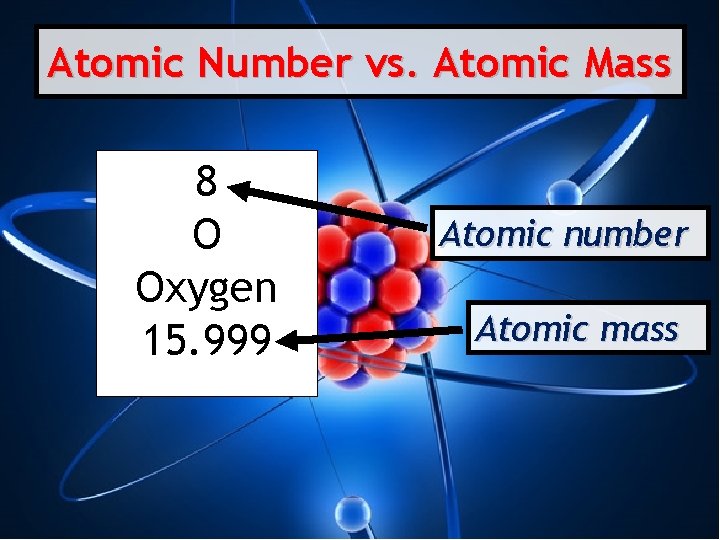
Atomic Number vs. Atomic Mass 8 O Oxygen 15. 999 Atomic number Atomic mass
Mass Number • Number of protons and neutrons combined. • It is always a whole number.

Finding the Number of Neutrons • Neutrons = mass # - atomic # • Example: Oxygen’s mass number is 16 and its atomic number is 8. The number of neutrons will be 16 -8 = 8.
Isotopes • Atoms with the same number of protons, but different number of neutrons. • They are chemically alike. • Have different mass numbers. • Written like this: Element–Mass Number
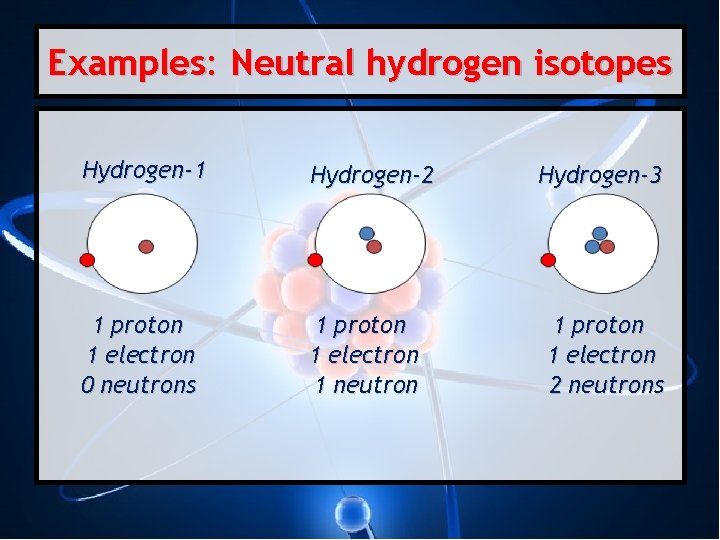
Examples: Neutral hydrogen isotopes Hydrogen-1 Hydrogen-2 1 proton 1 electron 0 neutrons 1 proton 1 electron 1 neutron Hydrogen-3 1 proton 1 electron 2 neutrons

Summary • Atomic # = protons = electrons (for neutral atoms) • Mass # = protons + neutrons • Neutrons = mass # - atomic # • Element Symbol (with mass # and Atomic #)
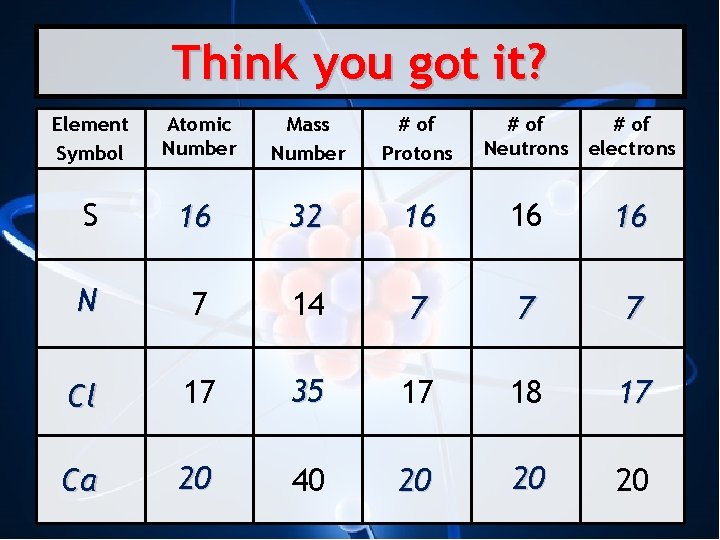
Think you got it? Element Symbol Atomic Number Mass Number # of Protons # of Neutrons # of electrons S 16 32 16 16 16 N 7 14 7 7 7 Cl 17 35 17 18 17 Ca 20 40 20 20 20
- Slides: 12