Unit 3 1 Chemical Reactions Sections 1 6



![Example 3. 15 Urea [(NH 2)2 CO] is prepared by reacting ammonia with carbon Example 3. 15 Urea [(NH 2)2 CO] is prepared by reacting ammonia with carbon](https://slidetodoc.com/presentation_image_h/0950465c2c19056db07b3c5c0691b848/image-17.jpg)

- Slides: 22

Unit 3. 1: Chemical Reactions Sections: 1. 6, 3. 7 -3. 10, 6. 2 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

Matter - anything that occupies space and has mass – measure of the quantity of matter SI unit of mass is the kilogram (kg) 1 kg = 1000 g = 1 x 103 g weight – force that gravity exerts on an object weight = c x mass A 1 kg bar will weigh on earth, c = 1. 0 1 kg on earth on moon, c ~ 0. 1 kg on moon 2

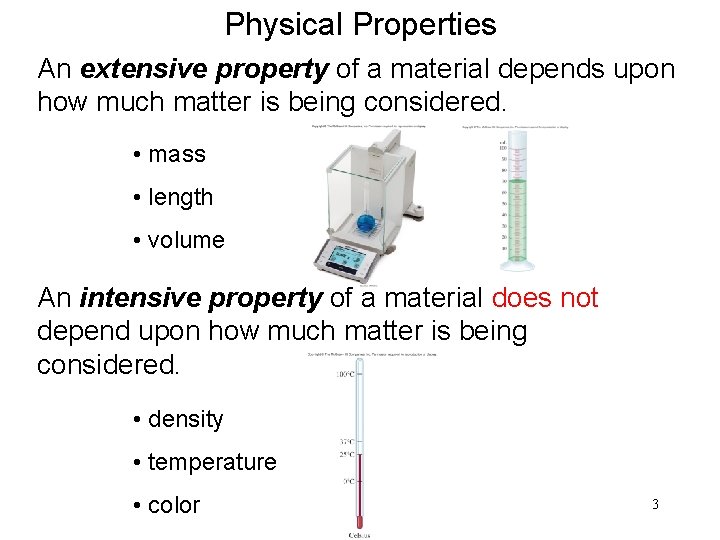
Physical Properties An extensive property of a material depends upon how much matter is being considered. • mass • length • volume An intensive property of a material does not depend upon how much matter is being considered. • density • temperature • color 3

Chemical Properties A chemical property describes the interaction of a substance with its surroundings (ability or inability) • heat of combustion • Enthalpy of formation • Toxicity • Chemical stability • flammability 4

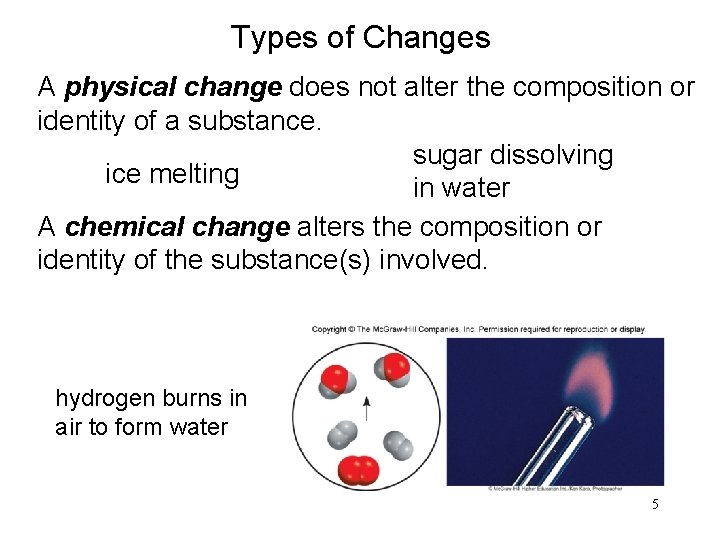
Types of Changes A physical change does not alter the composition or identity of a substance. sugar dissolving ice melting in water A chemical change alters the composition or identity of the substance(s) involved. hydrogen burns in air to form water 5

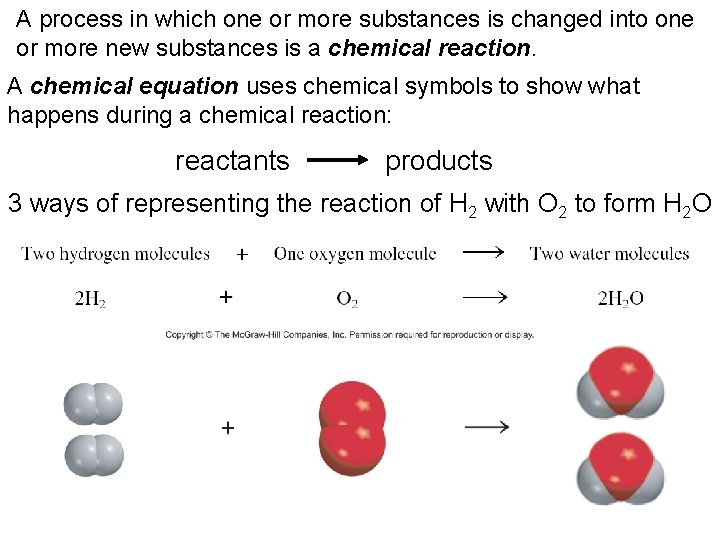
A process in which one or more substances is changed into one or more new substances is a chemical reaction. A chemical equation uses chemical symbols to show what happens during a chemical reaction: reactants products 3 ways of representing the reaction of H 2 with O 2 to form H 2 O 6

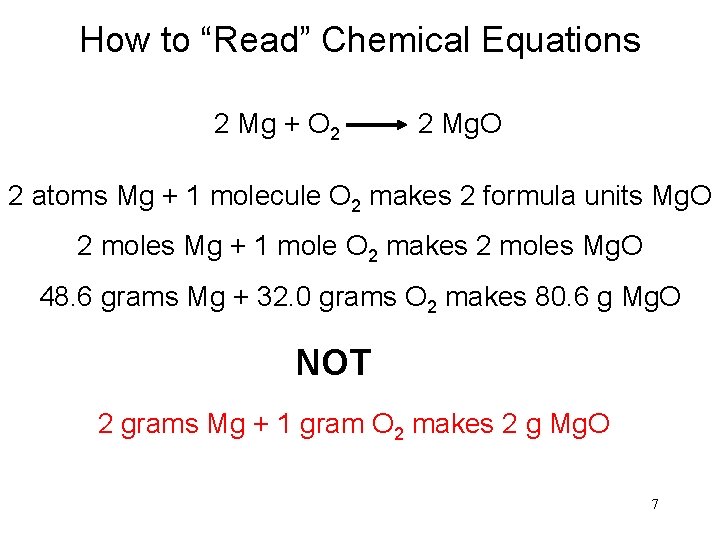
How to “Read” Chemical Equations 2 Mg + O 2 2 Mg. O 2 atoms Mg + 1 molecule O 2 makes 2 formula units Mg. O 2 moles Mg + 1 mole O 2 makes 2 moles Mg. O 48. 6 grams Mg + 32. 0 grams O 2 makes 80. 6 g Mg. O NOT 2 grams Mg + 1 gram O 2 makes 2 g Mg. O 7

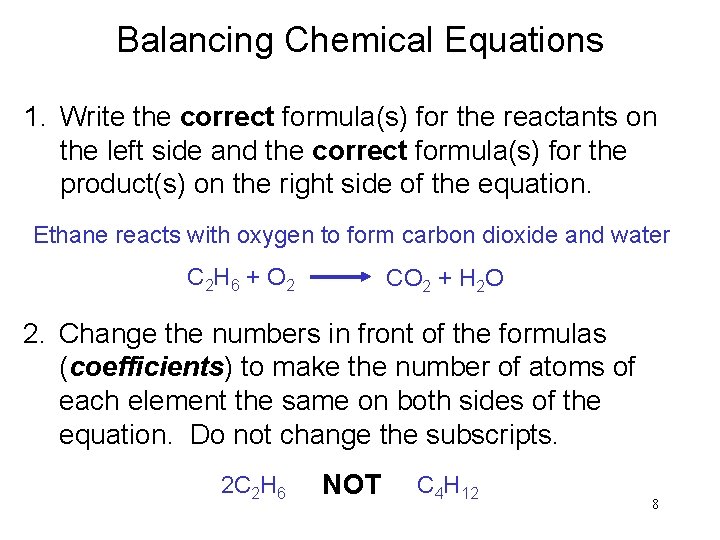
Balancing Chemical Equations 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide and water C 2 H 6 + O 2 CO 2 + H 2 O 2. Change the numbers in front of the formulas (coefficients) to make the number of atoms of each element the same on both sides of the equation. Do not change the subscripts. 2 C 2 H 6 NOT C 4 H 12 8

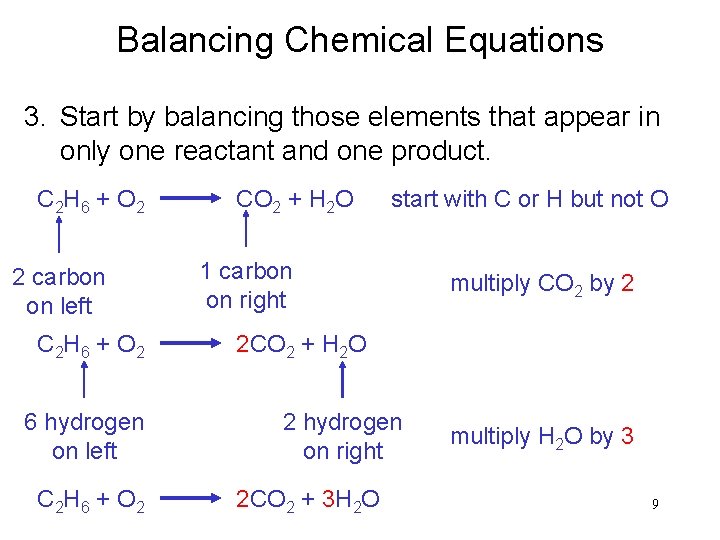
Balancing Chemical Equations 3. Start by balancing those elements that appear in only one reactant and one product. C 2 H 6 + O 2 2 carbon on left C 2 H 6 + O 2 6 hydrogen on left C 2 H 6 + O 2 CO 2 + H 2 O start with C or H but not O 1 carbon on right multiply CO 2 by 2 2 CO 2 + H 2 O 2 hydrogen on right 2 CO 2 + 3 H 2 O multiply H 2 O by 3 9

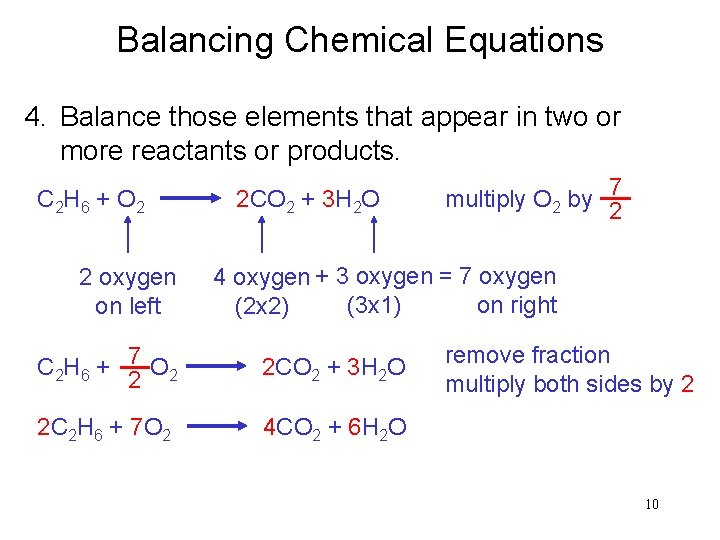
Balancing Chemical Equations 4. Balance those elements that appear in two or more reactants or products. C 2 H 6 + O 2 2 oxygen on left 2 CO 2 + 3 H 2 O multiply O 2 by 7 2 4 oxygen + 3 oxygen = 7 oxygen (3 x 1) on right (2 x 2) 7 C 2 H 6 + O 2 2 2 CO 2 + 3 H 2 O 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O remove fraction multiply both sides by 2 10

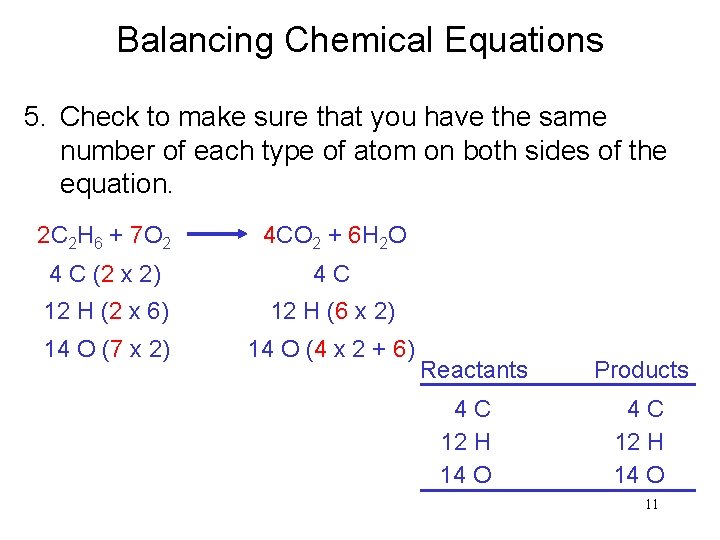
Balancing Chemical Equations 5. Check to make sure that you have the same number of each type of atom on both sides of the equation. 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O 4 C (2 x 2) 4 C 12 H (2 x 6) 12 H (6 x 2) 14 O (7 x 2) 14 O (4 x 2 + 6) Reactants 4 C 12 H 14 O Products 4 C 12 H 14 O 11

Example 3. 12 When aluminum metal is exposed to air, a protective layer of aluminum oxide (Al 2 O 3) forms on its surface. This layer prevents further reaction between aluminum and oxygen, and it is the reason that aluminum beverage cans do not corrode. [In the case of iron, the rust, or iron(III) oxide, that forms is too porous to protect the iron metal underneath, so rusting continues. ] Write a balanced equation for the formation of Al 2 O 3. An atomic scale image of aluminum oxide.

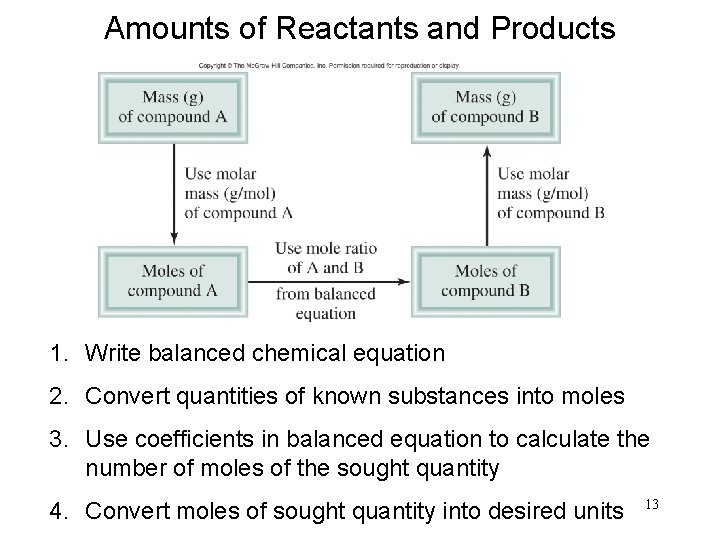
Amounts of Reactants and Products 1. Write balanced chemical equation 2. Convert quantities of known substances into moles 3. Use coefficients in balanced equation to calculate the number of moles of the sought quantity 4. Convert moles of sought quantity into desired units 13

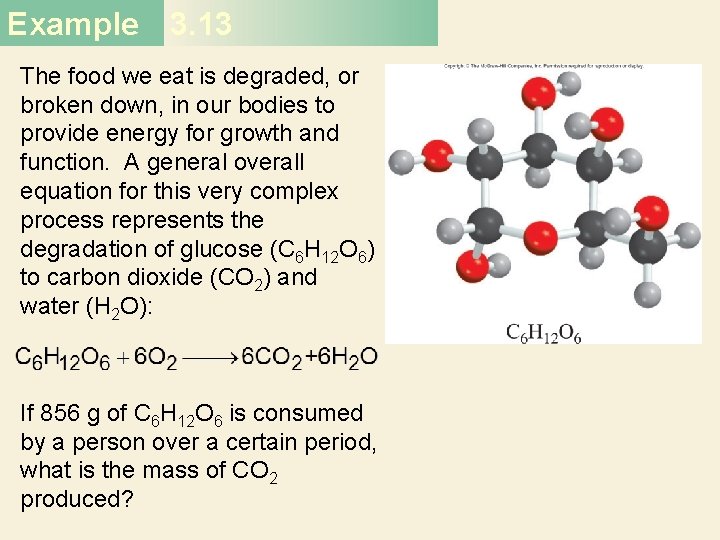
Example 3. 13 The food we eat is degraded, or broken down, in our bodies to provide energy for growth and function. A general overall equation for this very complex process represents the degradation of glucose (C 6 H 12 O 6) to carbon dioxide (CO 2) and water (H 2 O): If 856 g of C 6 H 12 O 6 is consumed by a person over a certain period, what is the mass of CO 2 produced?

Example 3. 14 All alkali metals react with water to produce hydrogen gas and the corresponding alkali metal hydroxide. A typical reaction is that between lithium and water: How many grams of Li are needed to produce 9. 89 g of H 2? Lithium reacting with water to produce hydrogen gas.

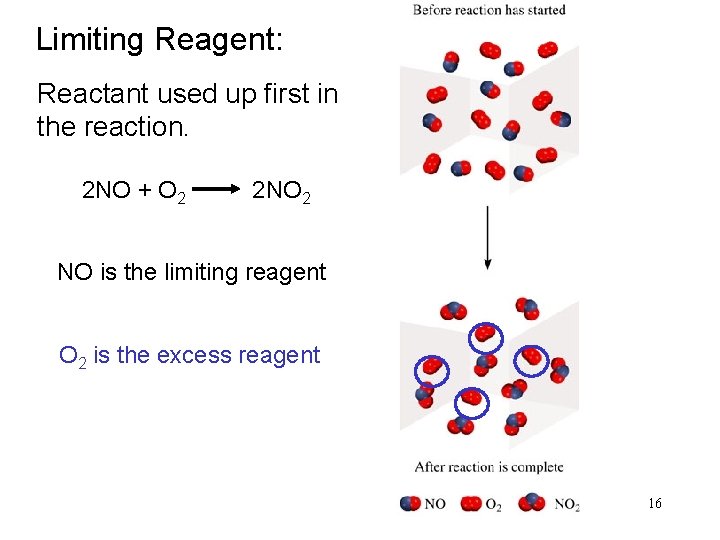
Limiting Reagent: Reactant used up first in the reaction. 2 NO + O 2 2 NO 2 NO is the limiting reagent O 2 is the excess reagent 16
![Example 3 15 Urea NH 22 CO is prepared by reacting ammonia with carbon Example 3. 15 Urea [(NH 2)2 CO] is prepared by reacting ammonia with carbon](https://slidetodoc.com/presentation_image_h/0950465c2c19056db07b3c5c0691b848/image-17.jpg)
Example 3. 15 Urea [(NH 2)2 CO] is prepared by reacting ammonia with carbon dioxide: In one process, 637. 2 g of NH 3 are treated with 1142 g of CO 2. (a) Which of the two reactants is the limiting reagent? (b) Calculate the mass of (NH 2)2 CO formed. (c) How much excess reagent (in grams) is left at the end of the reaction?

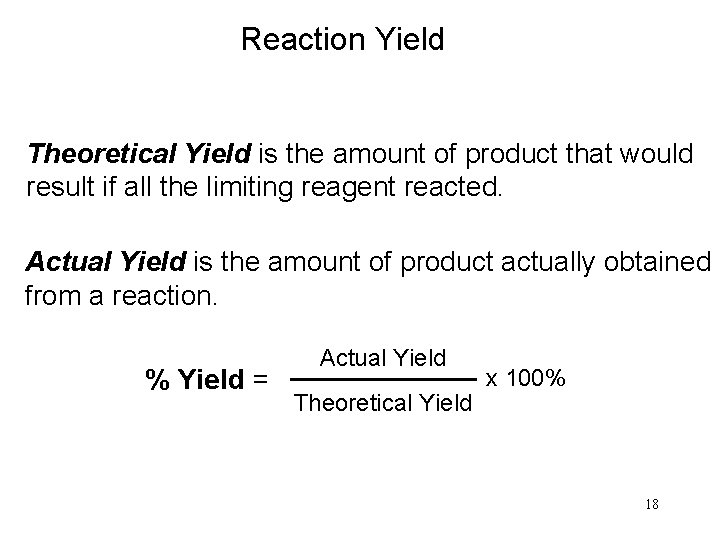
Reaction Yield Theoretical Yield is the amount of product that would result if all the limiting reagent reacted. Actual Yield is the amount of product actually obtained from a reaction. % Yield = Actual Yield Theoretical Yield x 100% 18

Example 3. 17 Titanium is a strong, lightweight, corrosion-resistant metal that is used in rockets, aircraft, jet engines, and bicycle frames. It is prepared by the reaction of titanium(IV) chloride with molten magnesium between 950°C and 1150°C: In a certain industrial operation 3. 54 × 107 g of Ti. Cl 4 are reacted with 1. 13 × 107 g of Mg. (a)Calculate theoretical yield of Ti in grams. (b)Calculate the percent yield if 7. 91 × 106 g of Ti are actually obtained.

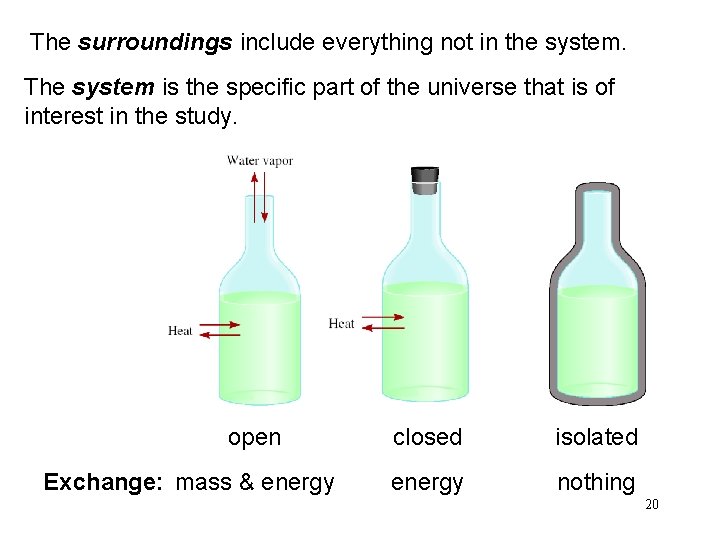
The surroundings include everything not in the system. The system is the specific part of the universe that is of interest in the study. open Exchange: mass & energy closed isolated energy nothing 20

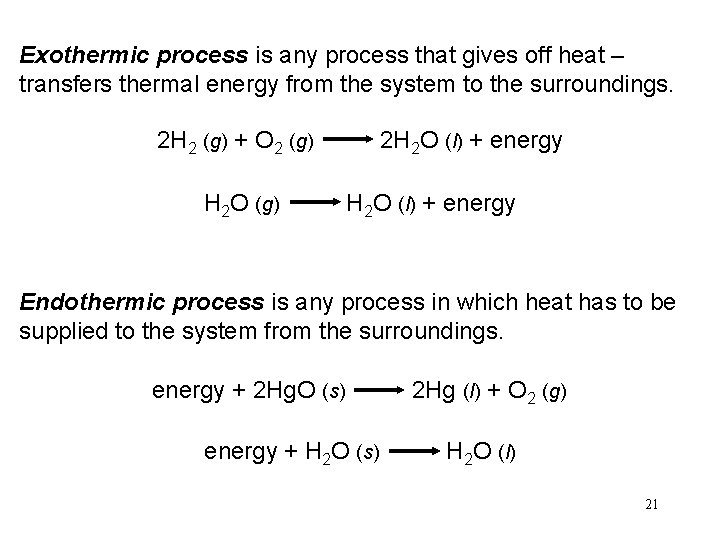
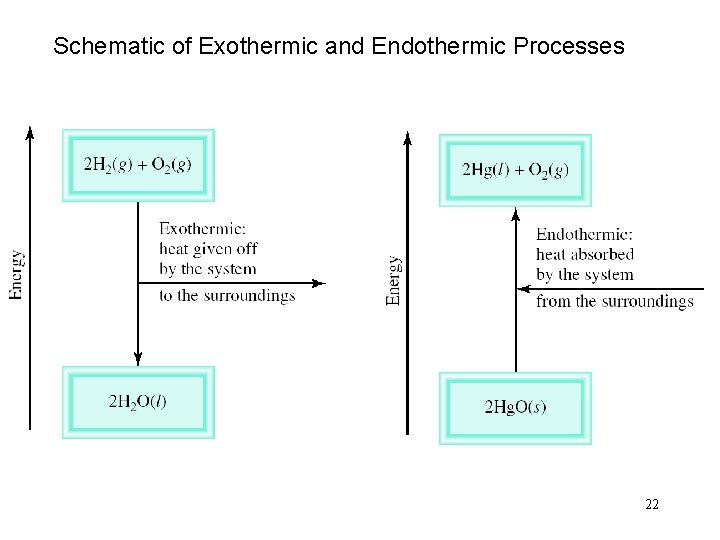
Exothermic process is any process that gives off heat – transfers thermal energy from the system to the surroundings. 2 H 2 (g) + O 2 (g) 2 H 2 O (l) + energy H 2 O (g) H 2 O (l) + energy Endothermic process is any process in which heat has to be supplied to the system from the surroundings. energy + 2 Hg. O (s) 2 Hg (l) + O 2 (g) energy + H 2 O (s) H 2 O (l) 21

Schematic of Exothermic and Endothermic Processes 22