Unit 23 Atom Elements and Stoichiometry Calculations with





















- Slides: 45

Unit 2/3: Atom, Elements, and Stoichiometry Calculations with Chemical Formulas and Equations By: Ms. Buroker Scott High School

Isotopes • Isotopes: atoms of the same element with different masses • The different masses come from different numbers of neutrons • The number of protons always remains the same, since those give us the identity of the element • The atomic mass shown on the PT is the weighted average of all the isotopes of the element

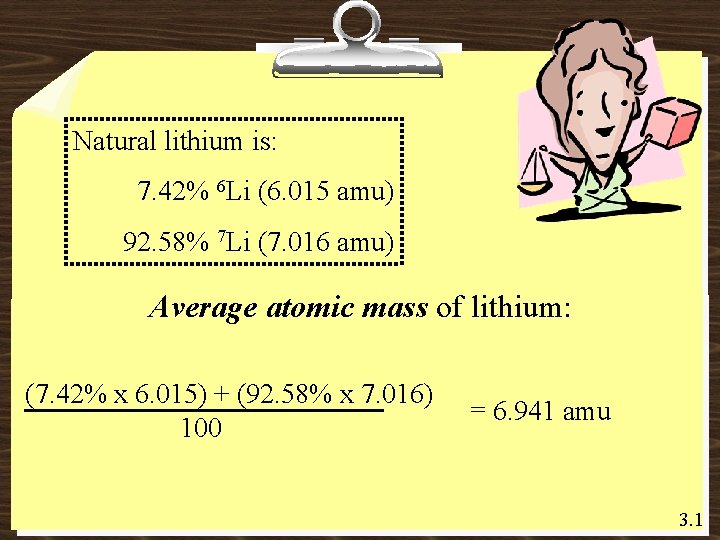
Natural lithium is: 7. 42% 6 Li (6. 015 amu) 92. 58% 7 Li (7. 016 amu) Average atomic mass of lithium: (7. 42% x 6. 015) + (92. 58% x 7. 016) 100 = 6. 941 amu 3. 1

So is there anything else that can change? • The protons of an element do not change • The neutrons of an element can change (isotopes) • The electrons of an element can also change • Ions: atoms of an element with a different amount of electrons than protons

Charged Atoms When electrons change, they create a charged atom, since the numbers of protons and electrons are now different • More electrons = negative ion (called an anion) • Less electrons = positive ion (called a cation)

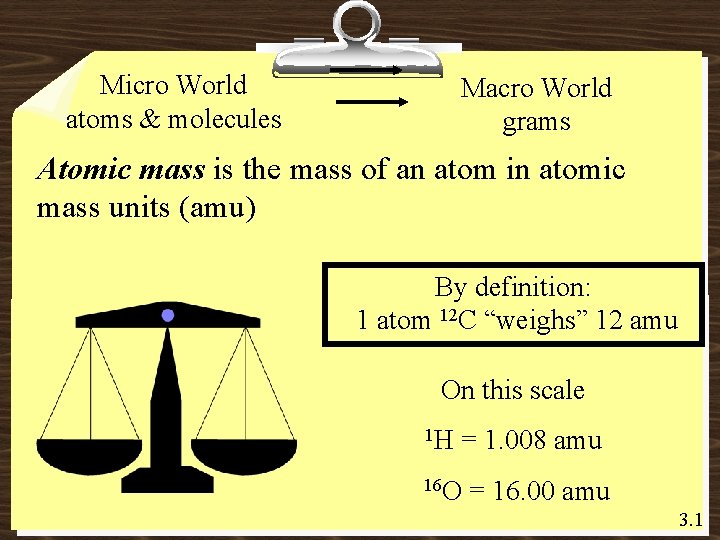
Micro World atoms & molecules Macro World grams Atomic mass is the mass of an atom in atomic mass units (amu) By definition: 1 atom 12 C “weighs” 12 amu On this scale 1 H = 1. 008 amu 16 O = 16. 00 amu 3. 1

First Things First!! The Law of Conservation of Mass!! Mass is neither created nor destroyed; therefore, chemical equations must be balanced in order to show equal numbers of moles of elements on both sides of the reaction!

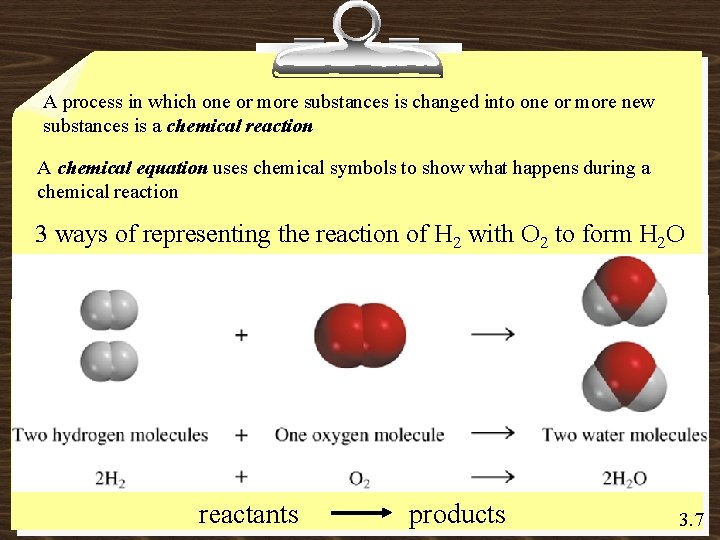
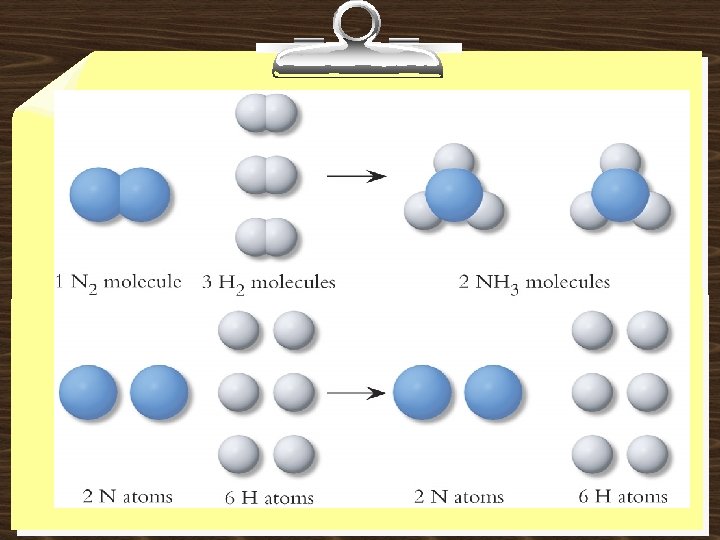
A process in which one or more substances is changed into one or more new substances is a chemical reaction A chemical equation uses chemical symbols to show what happens during a chemical reaction 3 ways of representing the reaction of H 2 with O 2 to form H 2 O reactants products 3. 7


How to “Read” Chemical Equations 2 Mg + O 2 2 Mg. O 2 atoms Mg + 1 molecule O 2 makes 2 formula units Mg. O 2 moles Mg + 1 mole O 2 makes 2 moles Mg. O 48. 6 grams Mg + 32. 0 grams O 2 makes 80. 6 g Mg. O IS NOT 2 grams Mg + 1 gram O 2 makes 2 g Mg. O 3. 7

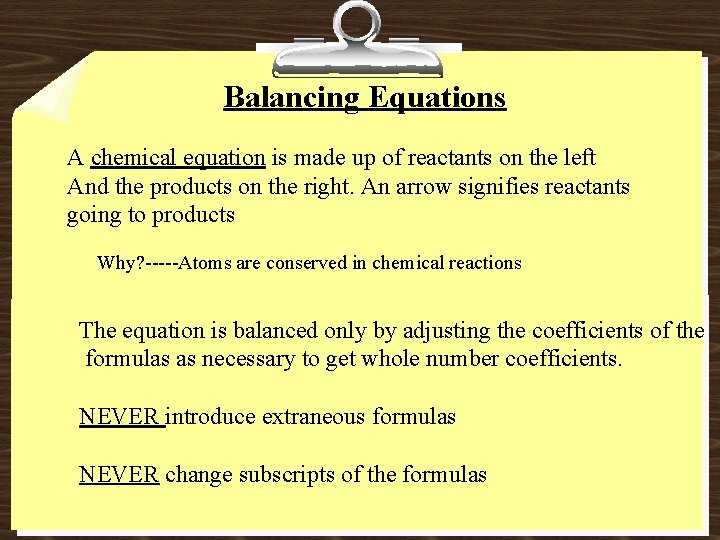
Balancing Equations A chemical equation is made up of reactants on the left And the products on the right. An arrow signifies reactants going to products Why? -----Atoms are conserved in chemical reactions The equation is balanced only by adjusting the coefficients of the formulas as necessary to get whole number coefficients. NEVER introduce extraneous formulas NEVER change subscripts of the formulas

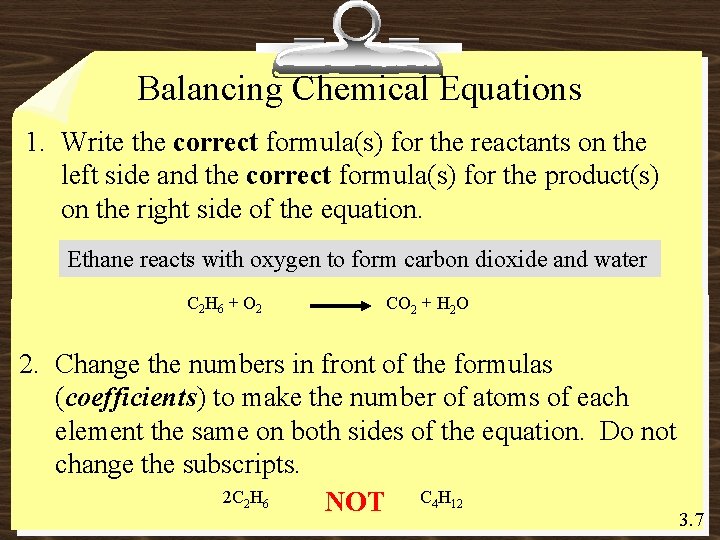
Balancing Chemical Equations 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide and water C 2 H 6 + O 2 CO 2 + H 2 O 2. Change the numbers in front of the formulas (coefficients) to make the number of atoms of each element the same on both sides of the equation. Do not change the subscripts. 2 C 2 H 6 NOT C 4 H 12 3. 7

Balancing Chemical Equations 3. Start by balancing those elements that appear in only one reactant and one product. C 2 H 6 + O 2 2 carbon on left C 2 H 6 + O 2 6 hydrogen on left C 2 H 6 + O 2 CO 2 + H 2 O 1 carbon on right start with C or H but not O multiply CO 2 by 2 2 CO 2 + H 2 O 2 hydrogen on right 2 CO 2 + 3 H 2 O multiply H 2 O by 3 3. 7

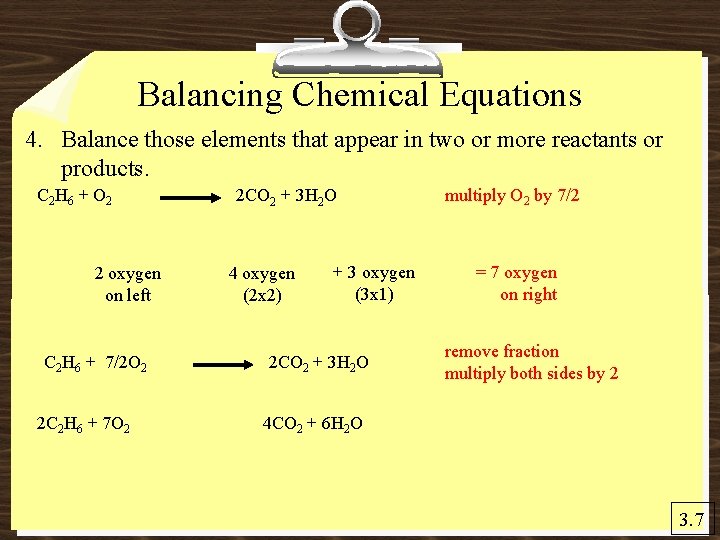
Balancing Chemical Equations 4. Balance those elements that appear in two or more reactants or products. C 2 H 6 + O 2 2 oxygen on left C 2 H 6 + 7/2 O 2 2 C 2 H 6 + 7 O 2 2 CO 2 + 3 H 2 O 4 oxygen (2 x 2) + 3 oxygen (3 x 1) 2 CO 2 + 3 H 2 O multiply O 2 by 7/2 = 7 oxygen on right remove fraction multiply both sides by 2 4 CO 2 + 6 H 2 O 3. 7

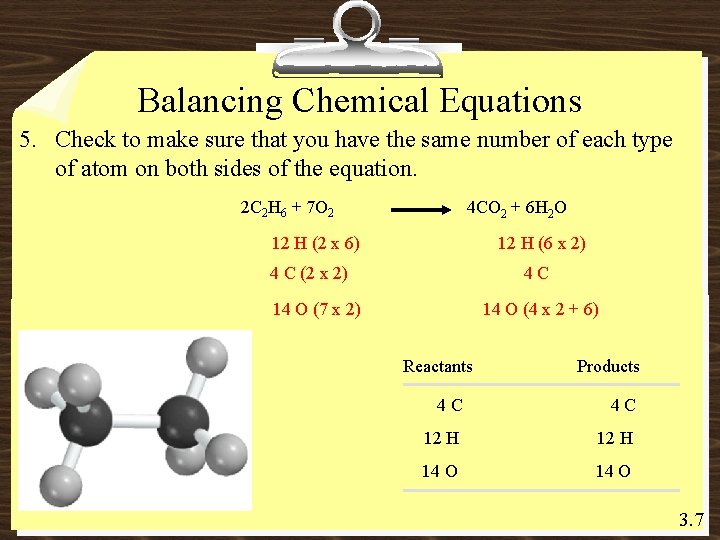
Balancing Chemical Equations 5. Check to make sure that you have the same number of each type of atom on both sides of the equation. 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O 12 H (2 x 6) 12 H (6 x 2) 4 C (2 x 2) 4 C 14 O (7 x 2) 14 O (4 x 2 + 6) Reactants Products 4 C 4 C 12 H 14 O 3. 7

Let’s Take a Little Pre-Test Get a Separate Piece of Paper and do the Best you can!

What is Chemical Stoichiometry? Stoichiometry is simply the study of the quantities of materials consumed and produced in chemical reactions.

Counting by Weighing Suppose you work in a candy store that sells jelly beans by the bean. Customers come in and order 50, 200, or 500 jelly beans. Counting these out by hand would be horrible! You (being the smart people that you are) decide to buy a scale and count the jelly beans by weighing them.

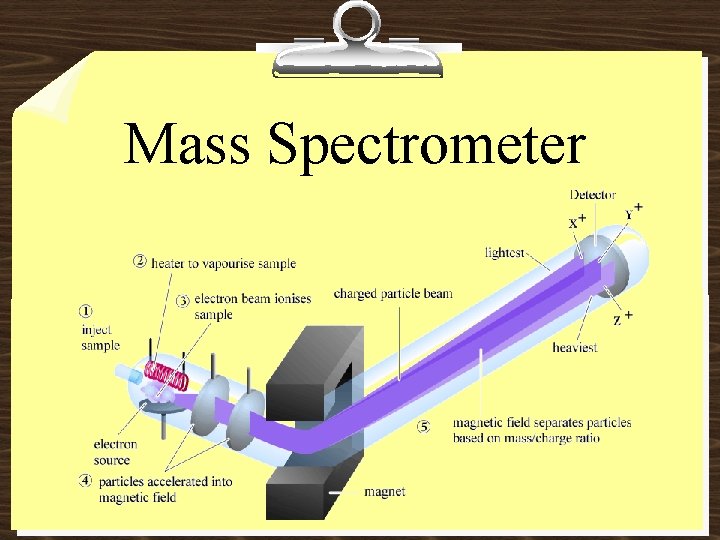
• Atoms are very small; therefore, we deal with samples of matter that contain huge numbers of atoms. So, we determine the number of atoms in a given sample by finding its mass. We need to know that average mass of the particular atom!

Atomic Mass The modern system for atomic mass is based on 12 C … 12 C is assigned a mass of exactly 12 atomic mass units (amu)

Mass Spectrometer

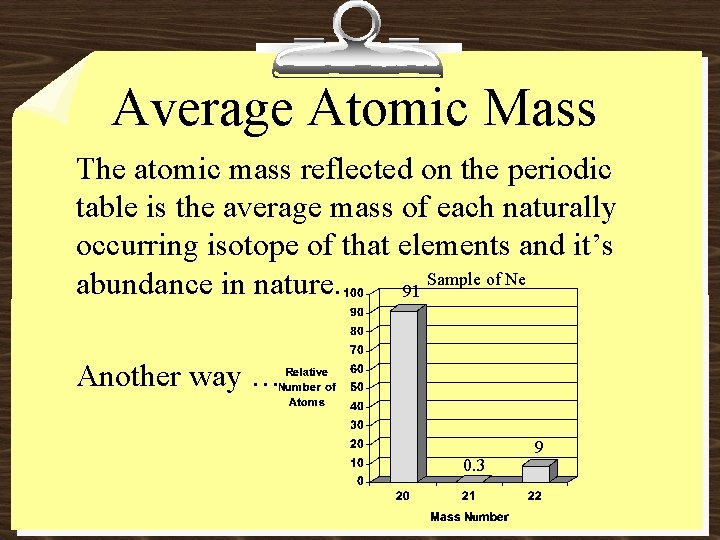
Average Atomic Mass The atomic mass reflected on the periodic table is the average mass of each naturally occurring isotope of that elements and it’s Sample of Ne abundance in nature. 91 Another way … 0. 3 9

The Mole We use the mole as our unit of measurement in determining the number of atoms … One mole (abbreviated mol) is the number of carbon atoms in exactly 12 g of pure 12 C = 6. 02 X 1023 Avogadro’s Number So …. One mole of any element (6. 02 X 1023 atoms) is equal to it’s atomic mass!!!!

Sample Problem Cobalt (Co) is a metal that is added to steel to improve its resistance to corrosion. Calculate both the number of moles in a sample of cobalt containing 5. 00 x 1020 atoms and the mass of the sample.

Molar mass is the mass of 1 mole of atoms in grams 1 mole 12 C atoms = 6. 022 x 1023 atoms = 12. 00 g 1 12 C atom = 12. 00 amu 1 mole 12 C atoms = 12. 00 g 12 C 1 mole lithium atoms = 6. 941 g of Li For any element atomic mass (amu) = molar mass (grams) 3. 2

C One Mole of: S Hg Cu Fe 3. 2

Do You Understand Molar Mass? How many atoms are in 0. 551 g of potassium (K) ? 1 mol K = 39. 10 g K 1 mol K = 6. 022 x 1023 atoms K 1 mol K 6. 022 x 1023 atoms K x 0. 551 g K x 1 mol K 39. 10 g K = 8. 49 x 1021 atoms K 3. 2

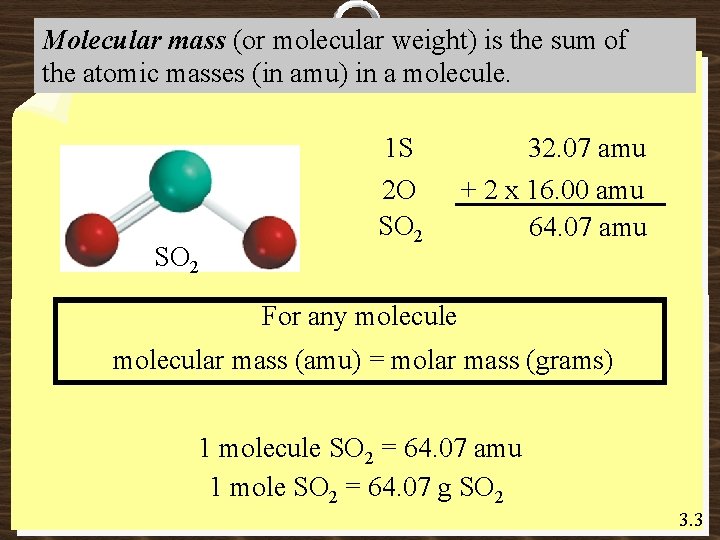
Molecular mass (or molecular weight) is the sum of the atomic masses (in amu) in a molecule. 1 S SO 2 2 O SO 2 32. 07 amu + 2 x 16. 00 amu 64. 07 amu For any molecule molecular mass (amu) = molar mass (grams) 1 molecule SO 2 = 64. 07 amu 1 mole SO 2 = 64. 07 g SO 2 3. 3

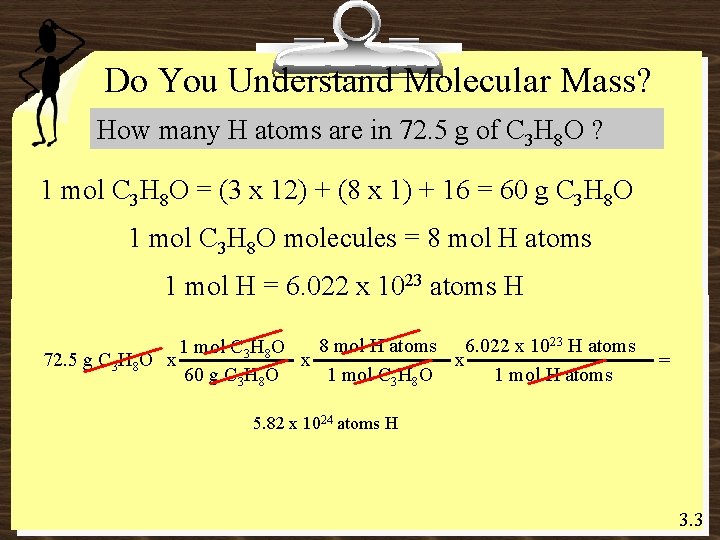
Do You Understand Molecular Mass? How many H atoms are in 72. 5 g of C 3 H 8 O ? 1 mol C 3 H 8 O = (3 x 12) + (8 x 1) + 16 = 60 g C 3 H 8 O 1 mol C 3 H 8 O molecules = 8 mol H atoms 1 mol H = 6. 022 x 1023 atoms H 8 mol H atoms 6. 022 x 1023 H atoms 1 mol C 3 H 8 O 72. 5 g C 3 H 8 O x x x 1 mol C 3 H 8 O 1 mol H atoms 60 g C 3 H 8 O = 5. 82 x 1024 atoms H 3. 3

Percent composition of an element in a compound = n x molar mass of element molar mass of compound x 100% n is the number of moles of the element in 1 mole of the compound %C = %H = %O = C 2 H 6 O 2 x (12. 01 g) 46. 07 g 6 x (1. 008 g) 46. 07 g 1 x (16. 00 g) 46. 07 g x 100% = 52. 14% x 100% = 13. 13% x 100% = 34. 73% 52. 14% + 13. 13% + 34. 73% = 100. 0% 3. 5

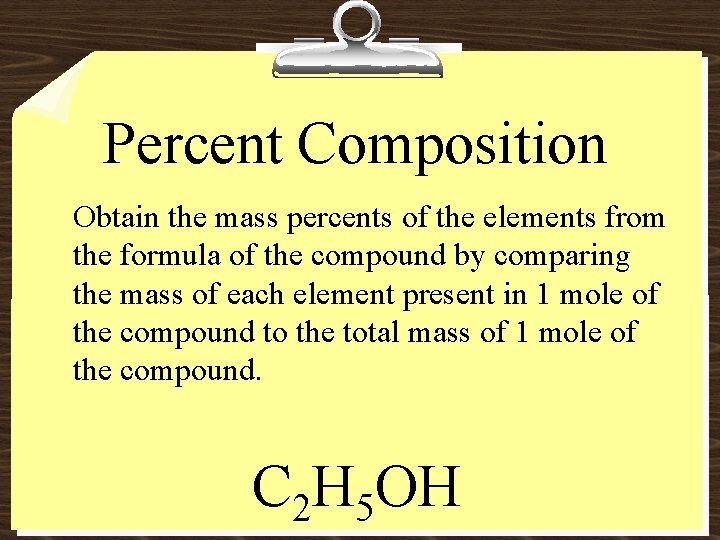
Percent Composition Obtain the mass percents of the elements from the formula of the compound by comparing the mass of each element present in 1 mole of the compound to the total mass of 1 mole of the compound. C 2 H 5 OH

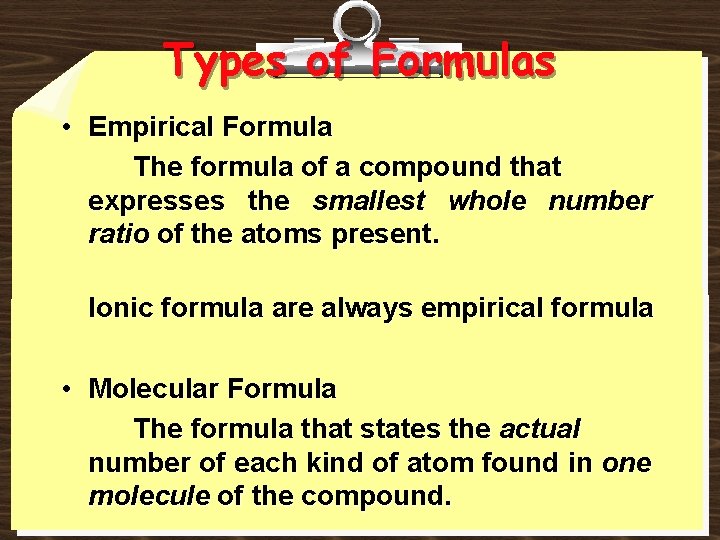
Types of Formulas • Empirical Formula The formula of a compound that expresses the smallest whole number ratio of the atoms present. Ionic formula are always empirical formula • Molecular Formula The formula that states the actual number of each kind of atom found in one molecule of the compound.

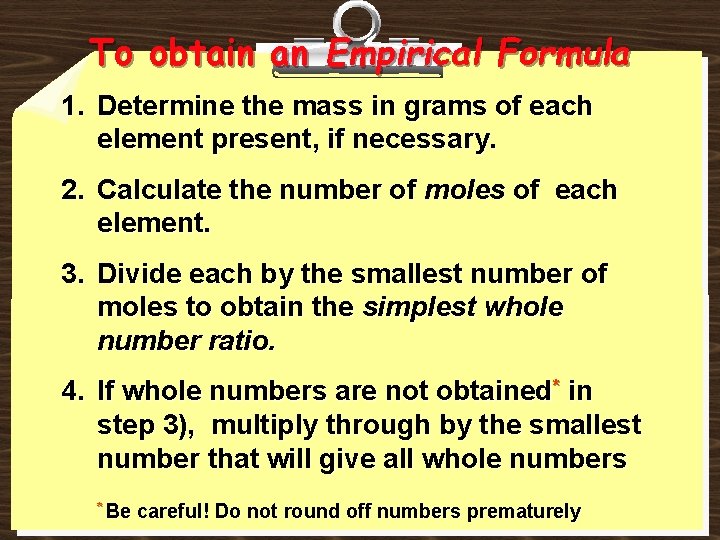
To obtain an Empirical Formula 1. Determine the mass in grams of each element present, if necessary. 2. Calculate the number of moles of each element. 3. Divide each by the smallest number of moles to obtain the simplest whole number ratio. 4. If whole numbers are not obtained* in step 3), multiply through by the smallest number that will give all whole numbers * Be careful! Do not round off numbers prematurely

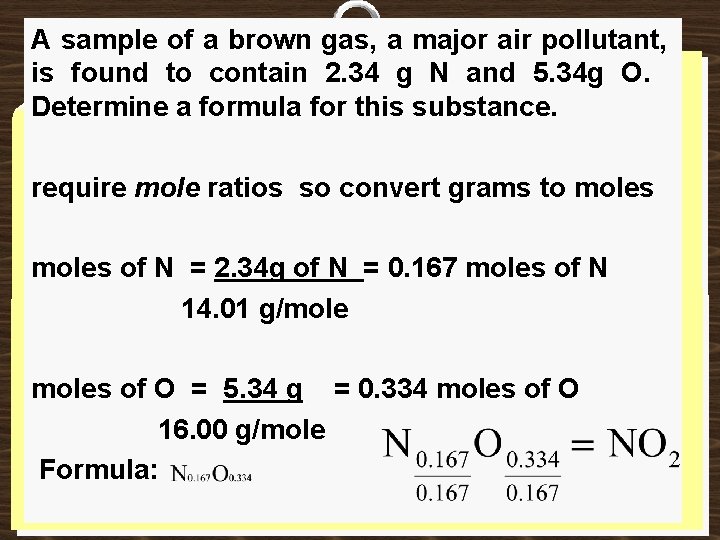
A sample of a brown gas, a major air pollutant, is found to contain 2. 34 g N and 5. 34 g O. Determine a formula for this substance. require mole ratios so convert grams to moles of N = 2. 34 g of N = 0. 167 moles of N 14. 01 g/moles of O = 5. 34 g = 0. 334 moles of O 16. 00 g/mole Formula:

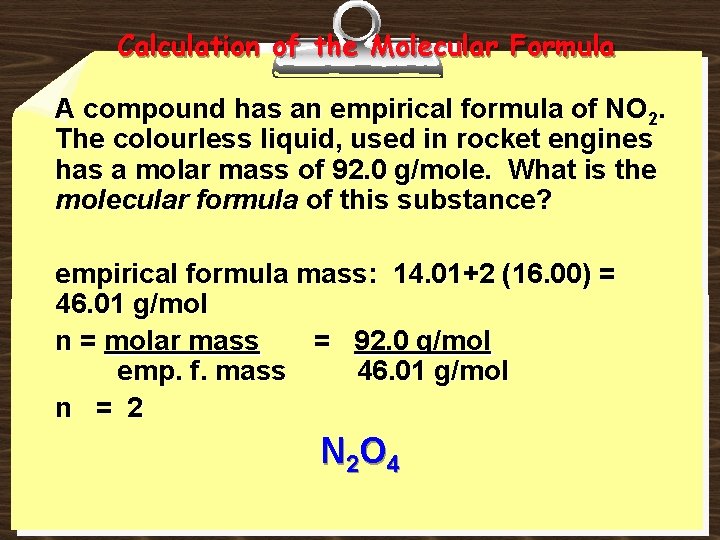
Calculation of the Molecular Formula A compound has an empirical formula of NO 2. The colourless liquid, used in rocket engines has a molar mass of 92. 0 g/mole. What is the molecular formula of this substance? empirical formula mass: 14. 01+2 (16. 00) = 46. 01 g/mol n = molar mass = 92. 0 g/mol emp. f. mass 46. 01 g/mol n = 2 N 2 O 4

Empirical Formula from % Composition A substance has the following composition by mass: 60. 80 % Na ; 28. 60 % B ; 10. 60 % H What is the empirical formula of the substance? Consider a sample size of 100 grams This will contain: 60. 80 grams of Na, 28. 60 grams of B, and 10. 60 grams H Determine the number of moles of each Determine the simplest whole number ratio

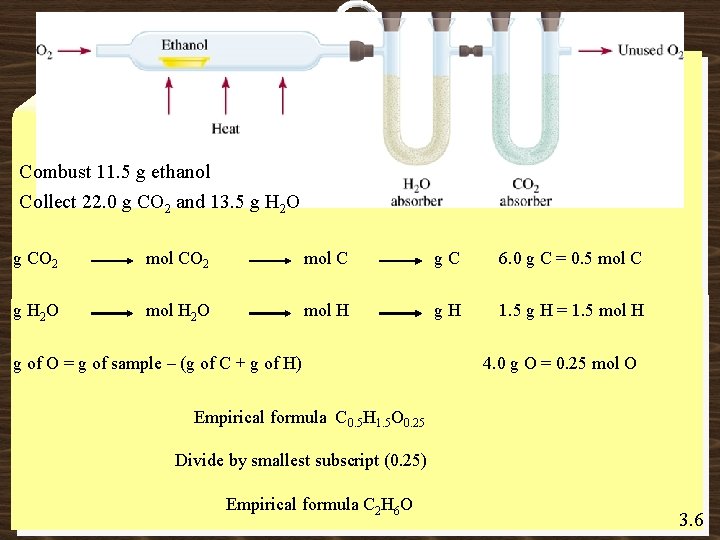
Combust 11. 5 g ethanol Collect 22. 0 g CO 2 and 13. 5 g H 2 O g CO 2 mol C g. C 6. 0 g C = 0. 5 mol C g H 2 O mol H 2 O mol H g. H 1. 5 g H = 1. 5 mol H g of O = g of sample – (g of C + g of H) 4. 0 g O = 0. 25 mol O Empirical formula C 0. 5 H 1. 5 O 0. 25 Divide by smallest subscript (0. 25) Empirical formula C 2 H 6 O 3. 6

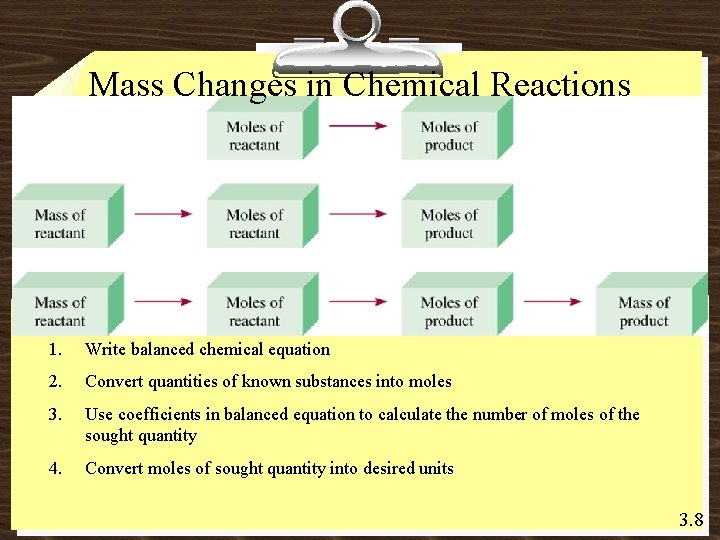
Mass Changes in Chemical Reactions 1. Write balanced chemical equation 2. Convert quantities of known substances into moles 3. Use coefficients in balanced equation to calculate the number of moles of the sought quantity 4. Convert moles of sought quantity into desired units 3. 8

Other units • Molarity • Moles solute / L solution • Gases • 22. 4 L = 1 mole of ANY GAS at STP

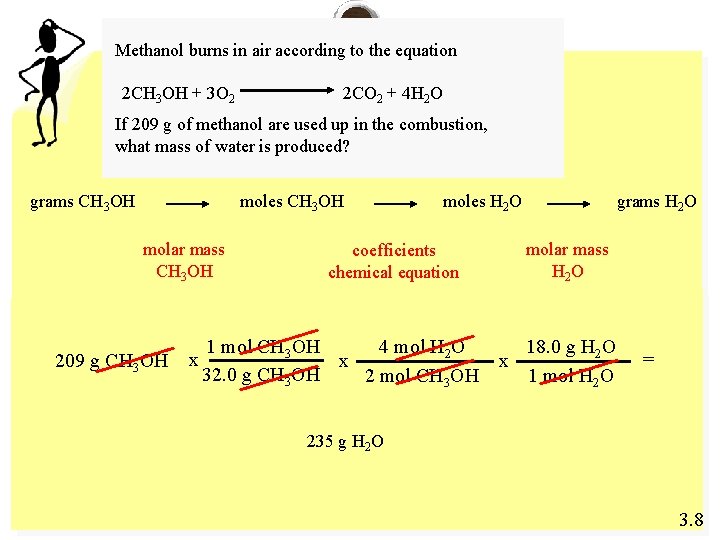
Methanol burns in air according to the equation 2 CH 3 OH + 3 O 2 2 CO 2 + 4 H 2 O If 209 g of methanol are used up in the combustion, what mass of water is produced? grams CH 3 OH moles CH 3 OH molar mass CH 3 OH 209 g CH 3 OH x moles H 2 O molar mass H 2 O coefficients chemical equation 4 mol H 2 O 1 mol CH 3 OH x 32. 0 g CH 3 OH 2 mol CH 3 OH grams H 2 O x 18. 0 g H 2 O 1 mol H 2 O = 235 g H 2 O 3. 8

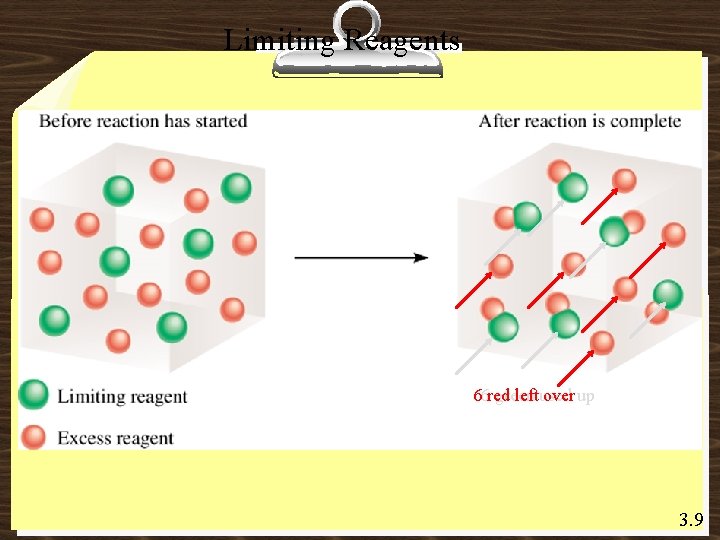
Limiting Reagents 66 red green leftused over up 3. 9

Method 1 • • Pick A Product Try ALL the reactants The lowest answer will be the correct answer The reactant that gives the lowest answer will be the limiting reactant

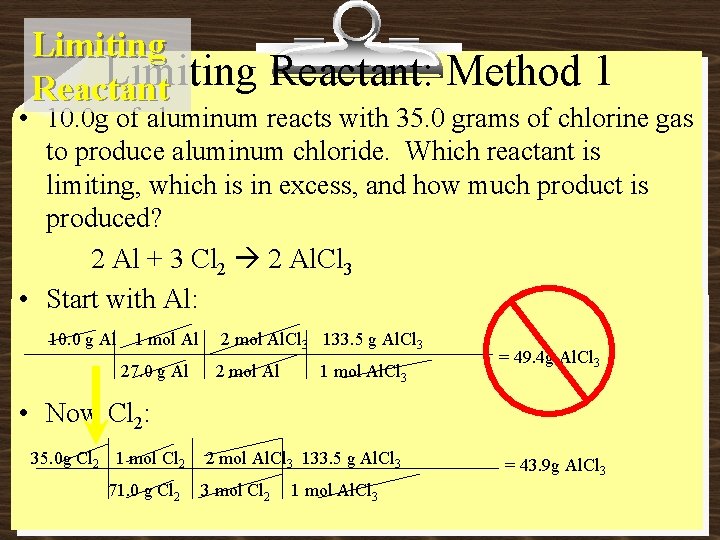
Limiting Reactant: Method 1 • 10. 0 g of aluminum reacts with 35. 0 grams of chlorine gas to produce aluminum chloride. Which reactant is limiting, which is in excess, and how much product is produced? 2 Al + 3 Cl 2 2 Al. Cl 3 • Start with Al: 10. 0 g Al 1 mol Al 27. 0 g Al 2 mol Al. Cl 3 133. 5 g Al. Cl 3 2 mol Al 1 mol Al. Cl 3 = 49. 4 g Al. Cl 3 • Now Cl 2: 35. 0 g Cl 2 1 mol Cl 2 71. 0 g Cl 2 2 mol Al. Cl 3 133. 5 g Al. Cl 3 3 mol Cl 2 1 mol Al. Cl 3 = 43. 9 g Al. Cl 3

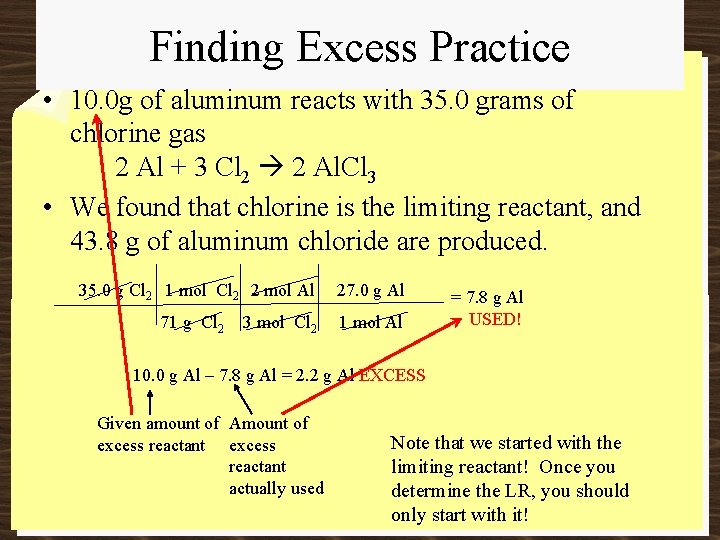
Finding Excess Practice • 10. 0 g of aluminum reacts with 35. 0 grams of chlorine gas 2 Al + 3 Cl 2 2 Al. Cl 3 • We found that chlorine is the limiting reactant, and 43. 8 g of aluminum chloride are produced. 35. 0 g Cl 2 1 mol Cl 2 2 mol Al 27. 0 g Al 71 g Cl 2 1 mol Al 3 mol Cl 2 = 7. 8 g Al USED! 10. 0 g Al – 7. 8 g Al = 2. 2 g Al EXCESS Given amount of Amount of excess reactant actually used Note that we started with the limiting reactant! Once you determine the LR, you should only start with it!

Theoretical Yield is the amount of product that would result if all the limiting reagent reacted. Actual Yield is the amount of product actually obtained from a reaction. % Yield = Actual Yield x 100 Theoretical Yield 3. 10