UNIT 2 VOCABULARY BIOLOGY CHAPTER 6 THE CHEMISTRY

UNIT 2 VOCABULARY BIOLOGY

CHAPTER 6 – THE CHEMISTRY OF LIFE 1. 2. 3. 4. 5. 6. 7. 8. Elements Compound Metabolism p. H Acid Base Isomer monomer 9. Polymer 10. Carbohydrate 11. Lipid 12. Protein 13. Amino acid 14. Peptide bond 15. Enzyme 16. Nucleic acid 17. Nucleotide
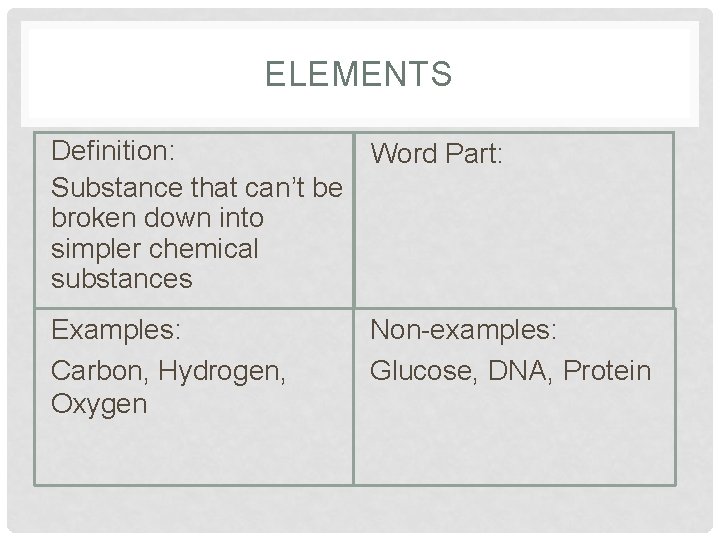
ELEMENTS Definition: Word Part: Substance that can’t be broken down into simpler chemical substances Examples: Carbon, Hydrogen, Oxygen Non-examples: Glucose, DNA, Protein
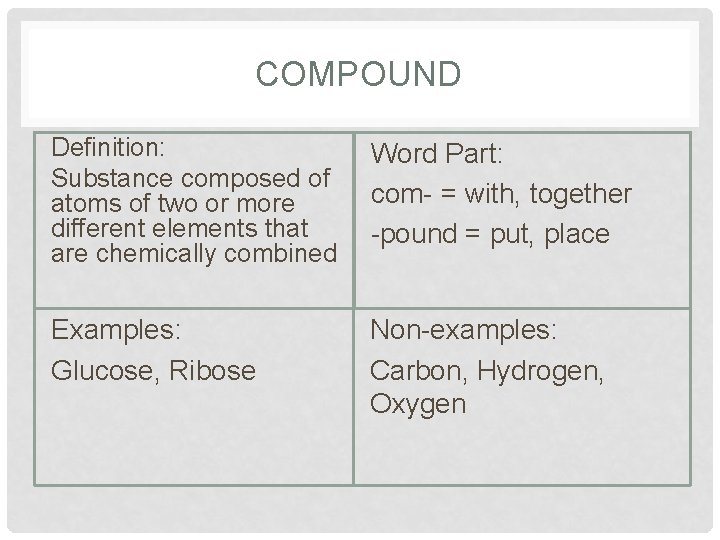
COMPOUND Definition: Substance composed of atoms of two or more different elements that are chemically combined Word Part: com- = with, together -pound = put, place Examples: Glucose, Ribose Non-examples: Carbon, Hydrogen, Oxygen
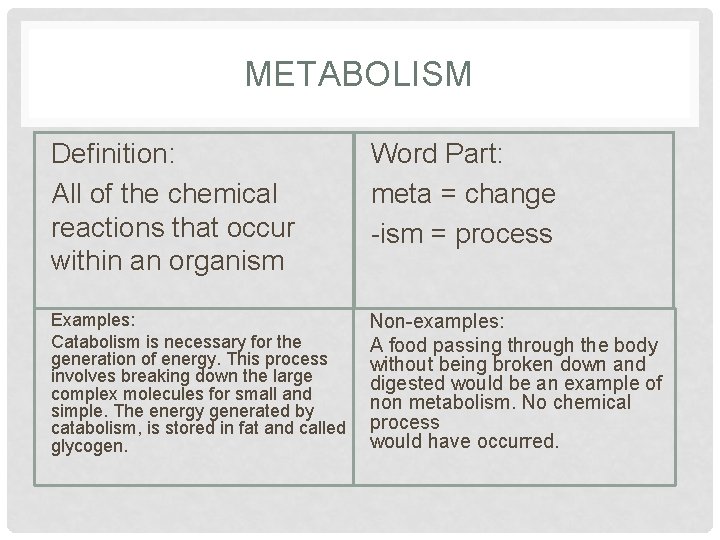
METABOLISM Definition: All of the chemical reactions that occur within an organism Word Part: meta = change -ism = process Examples: Catabolism is necessary for the generation of energy. This process involves breaking down the large complex molecules for small and simple. The energy generated by catabolism, is stored in fat and called glycogen. Non-examples: A food passing through the body without being broken down and digested would be an example of non metabolism. No chemical process would have occurred.
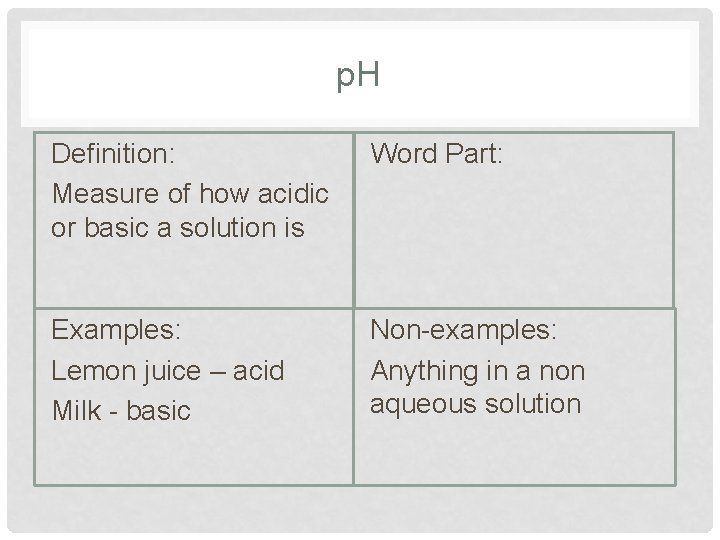
p. H Definition: Measure of how acidic or basic a solution is Word Part: Examples: Lemon juice – acid Milk - basic Non-examples: Anything in a non aqueous solution
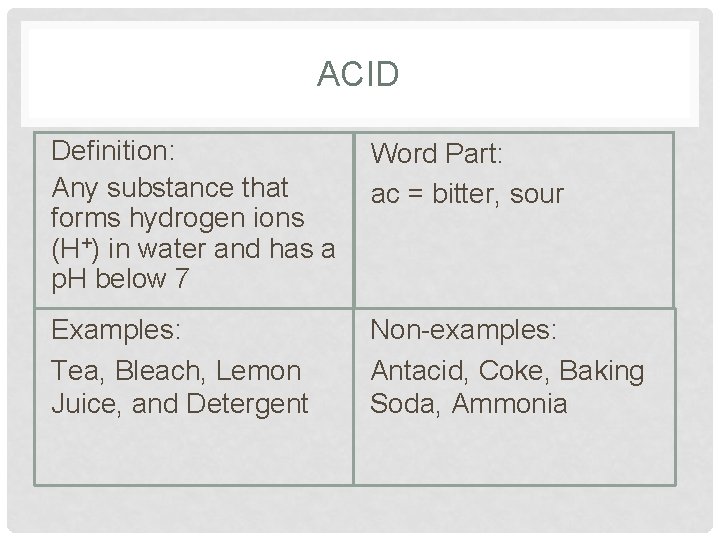
ACID Definition: Any substance that forms hydrogen ions (H+) in water and has a p. H below 7 Word Part: ac = bitter, sour Examples: Tea, Bleach, Lemon Juice, and Detergent Non-examples: Antacid, Coke, Baking Soda, Ammonia
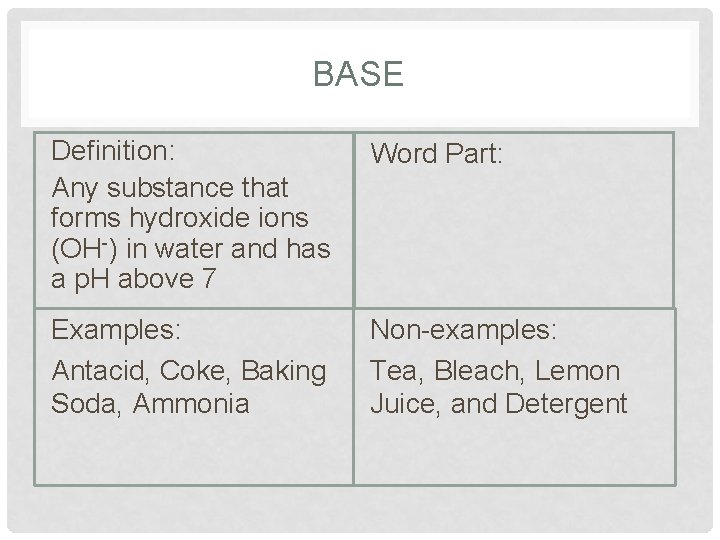
BASE Definition: Any substance that forms hydroxide ions (OH-) in water and has a p. H above 7 Word Part: Examples: Antacid, Coke, Baking Soda, Ammonia Non-examples: Tea, Bleach, Lemon Juice, and Detergent
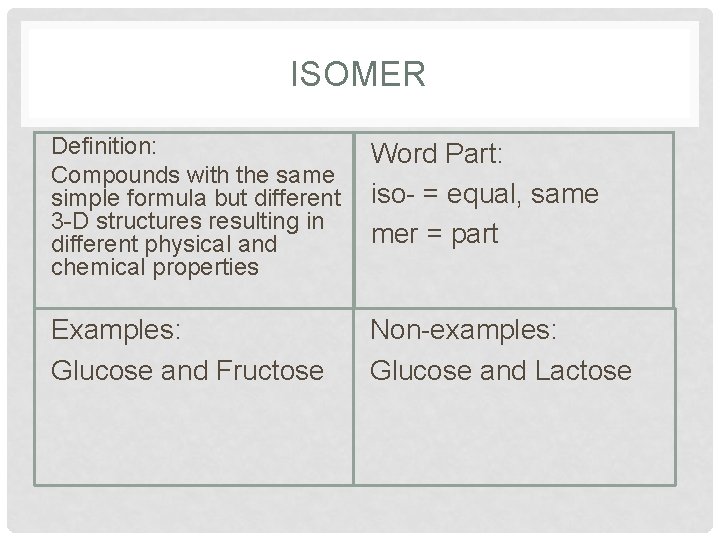
ISOMER Definition: Compounds with the same simple formula but different 3 -D structures resulting in different physical and chemical properties Word Part: iso- = equal, same mer = part Examples: Glucose and Fructose Non-examples: Glucose and Lactose
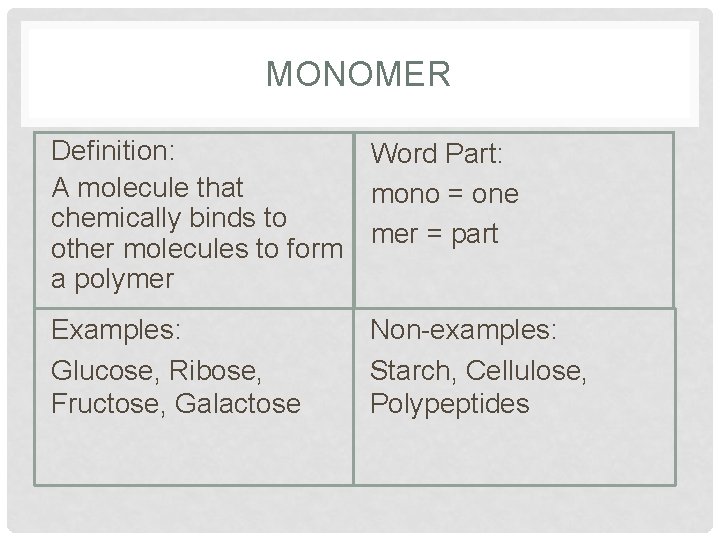
MONOMER Definition: Word Part: A molecule that mono = one chemically binds to mer = part other molecules to form a polymer Examples: Glucose, Ribose, Fructose, Galactose Non-examples: Starch, Cellulose, Polypeptides
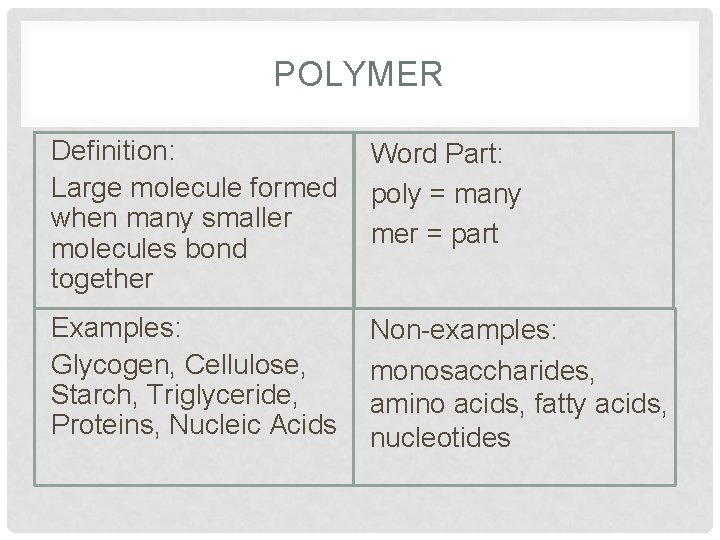
POLYMER Definition: Large molecule formed when many smaller molecules bond together Word Part: poly = many mer = part Examples: Glycogen, Cellulose, Starch, Triglyceride, Proteins, Nucleic Acids Non-examples: monosaccharides, amino acids, fatty acids, nucleotides
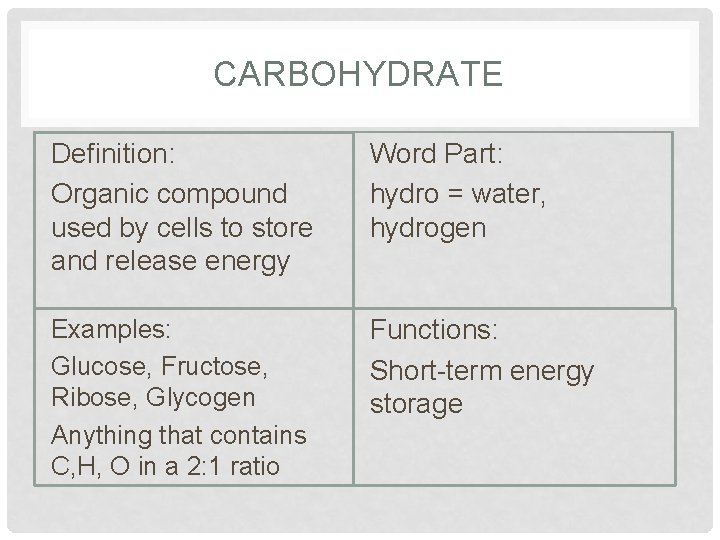
CARBOHYDRATE Definition: Organic compound used by cells to store and release energy Word Part: hydro = water, hydrogen Examples: Glucose, Fructose, Ribose, Glycogen Anything that contains C, H, O in a 2: 1 ratio Functions: Short-term energy storage
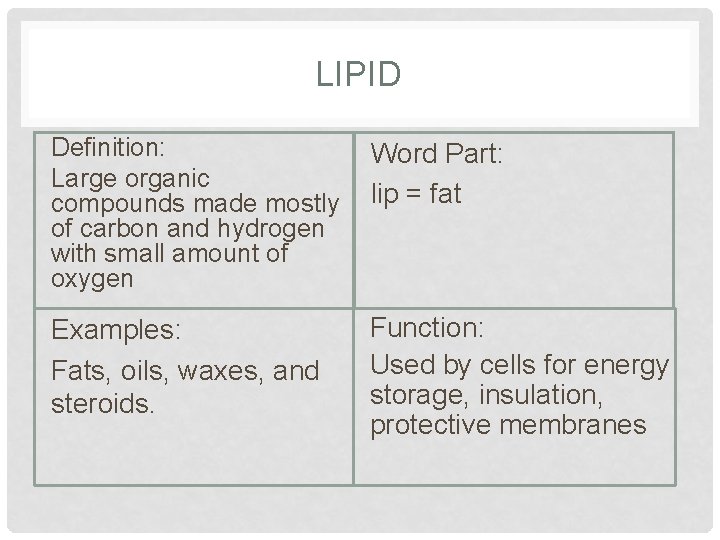
LIPID Definition: Large organic compounds made mostly of carbon and hydrogen with small amount of oxygen Word Part: lip = fat Examples: Fats, oils, waxes, and steroids. Function: Used by cells for energy storage, insulation, protective membranes
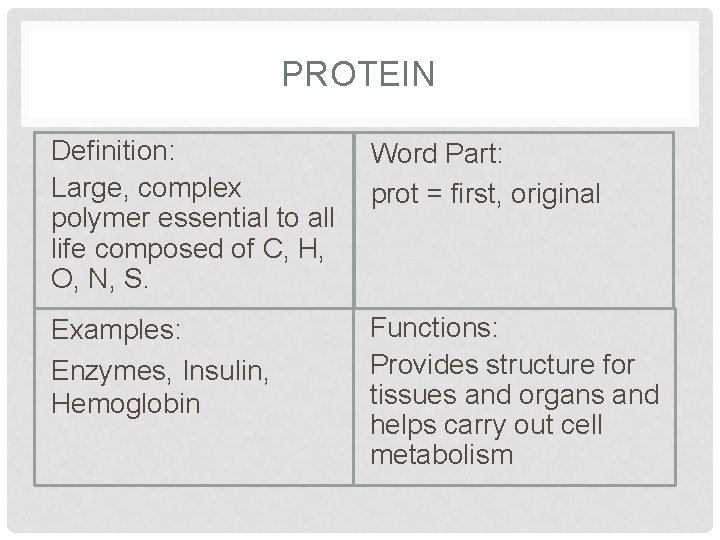
PROTEIN Definition: Large, complex polymer essential to all life composed of C, H, O, N, S. Word Part: prot = first, original Examples: Enzymes, Insulin, Hemoglobin Functions: Provides structure for tissues and organs and helps carry out cell metabolism
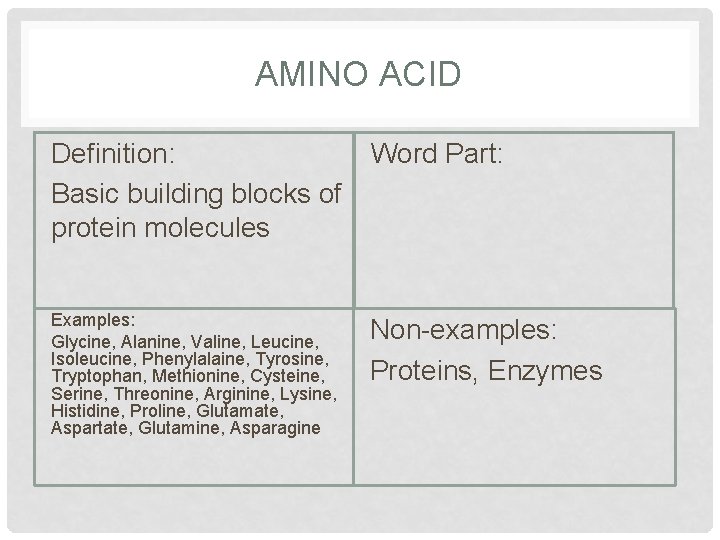
AMINO ACID Definition: Basic building blocks of protein molecules Word Part: Examples: Glycine, Alanine, Valine, Leucine, Isoleucine, Phenylalaine, Tyrosine, Tryptophan, Methionine, Cysteine, Serine, Threonine, Arginine, Lysine, Histidine, Proline, Glutamate, Aspartate, Glutamine, Asparagine Non-examples: Proteins, Enzymes
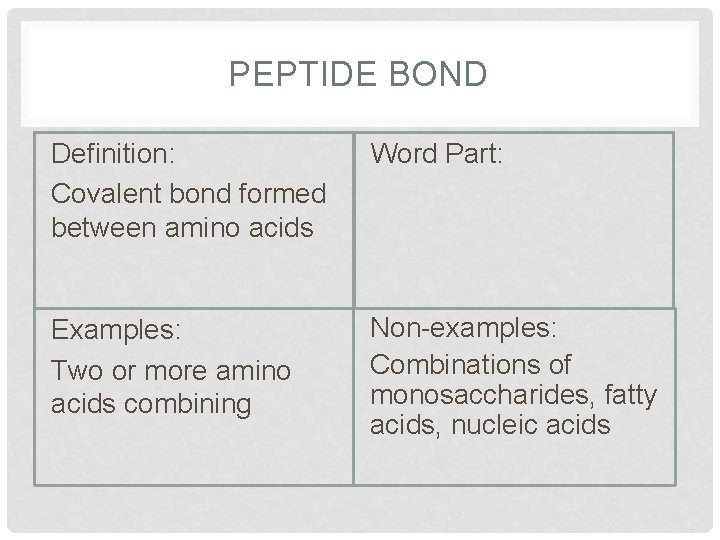
PEPTIDE BOND Definition: Covalent bond formed between amino acids Word Part: Examples: Two or more amino acids combining Non-examples: Combinations of monosaccharides, fatty acids, nucleic acids
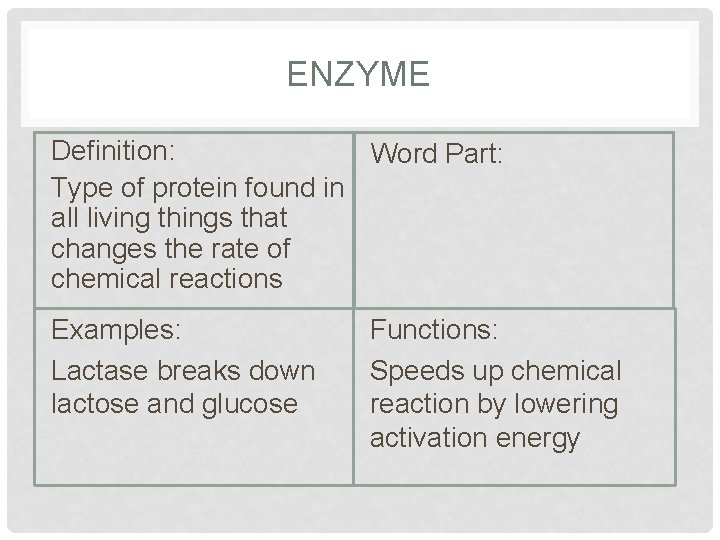
ENZYME Definition: Word Part: Type of protein found in all living things that changes the rate of chemical reactions Examples: Lactase breaks down lactose and glucose Functions: Speeds up chemical reaction by lowering activation energy
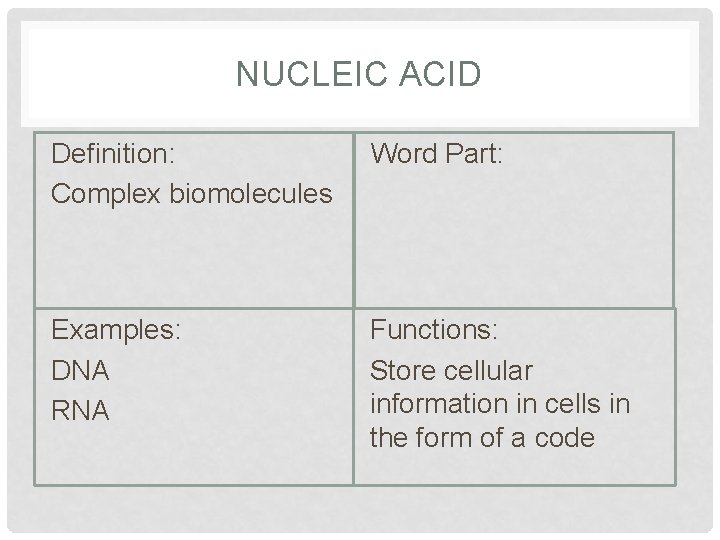
NUCLEIC ACID Definition: Complex biomolecules Word Part: Examples: DNA RNA Functions: Store cellular information in cells in the form of a code
NUCLEOTIDE Definition: Subunits of nucleic acid formed from a simple sugar, a phosphate group, and a nitrogenous base. Word Part: Examples: Adenine, Guanine, Thymine, Cytosine, Uracil Non-examples: DNA and RNA
- Slides: 19