Unit 2 Stoichiometry Mole Calculations Mole Bridge Average

Unit 2: Stoichiometry & Mole Calculations Mole Bridge & Average atomic mass
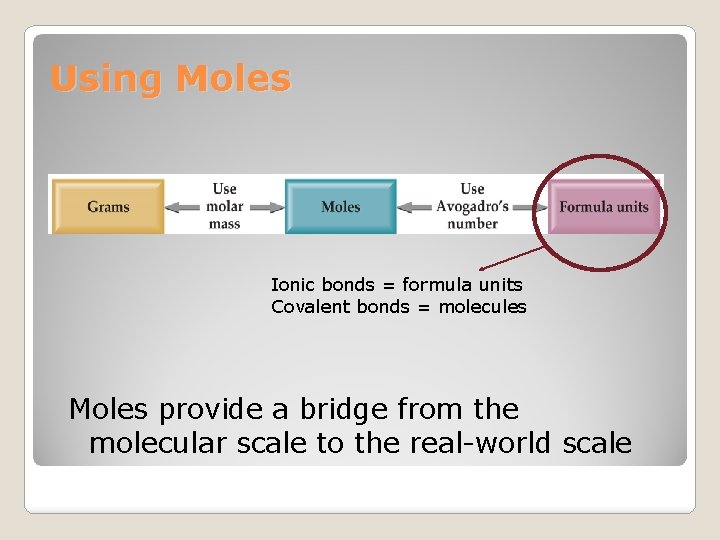
Using Moles Ionic bonds = formula units Covalent bonds = molecules Moles provide a bridge from the molecular scale to the real-world scale

SAMPLE EXERCISE 3. 10 Converting Grams to Moles Calculate the number of moles of glucose (C 6 H 12 O 6) in 5. 380 g of C 6 H 12 O 6. Plan: The molar mass of a substance provides the factor for converting grams to moles. The molar mass of C 6 H 12 O 6 is 180. 0 g/mol (Sample Exercise 3. 9). Solve: Using 1 mol C 6 H 12 O 6 = 180. 0 g C 6 H 12 O 6 to write the appropriate conversion factor, we have Check: Because 5. 380 g is less than the molar mass, it is reasonable that our answer is less than one mole. The units of our answer (mol) are appropriate. The original data had four significant figures, so our answer has four significant figures.

SAMPLE EXERCISE 3. 11 Converting Moles to Grams Calculate the mass, in grams, of 0. 433 mol of calcium nitrate. Plan: In order to convert moles to grams, we need the molar mass, which we can calculate using the chemical formula and atomic weights. Check: The number of moles is less than 1, so the number of grams must be less than the molar mass, 164. 1 g. Using rounded numbers to estimate, we have 0. 5 150 = 75 g. Thus, the magnitude of our answer is reasonable. Both the units (g) and the number of significant figures (3) are correct.

SAMPLE EXERCISE 3. 12 Calculating the Number of Molecules and Number of Atoms from Mass (a) How many glucose molecules are in 5. 23 g of C 6 H 12 O 6? (b) How many oxygen atoms are in this sample? Solve: Molecules C 6 H 12 O 6 Check: The magnitude of the answer is reasonable. Because the mass we began with is less than a mole, there should be less than 6. 02 1023 molecules. We can make a ballpark estimate of the answer: 5/200 = 2. 5 10– 2 mol; 2. 5 10– 2 6 1023 = 15 1021 = 1. 5 1022 molecules. The units (molecules) and significant figures (three) are appropriate.
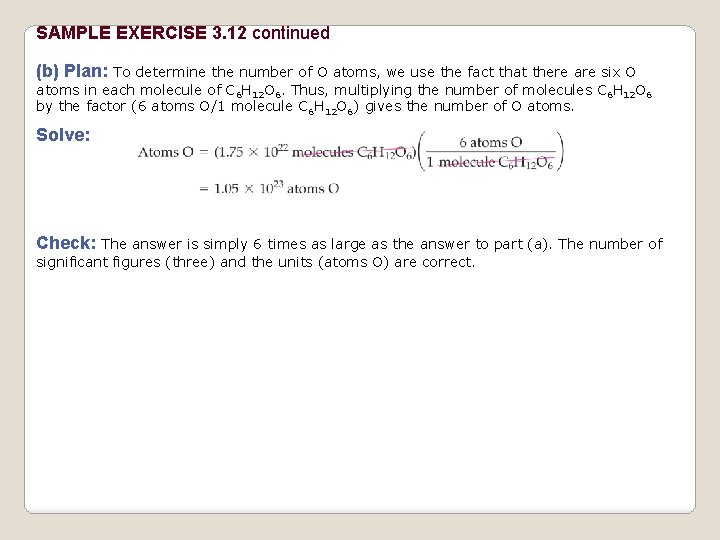
SAMPLE EXERCISE 3. 12 continued (b) Plan: To determine the number of O atoms, we use the fact that there are six O atoms in each molecule of C 6 H 12 O 6. Thus, multiplying the number of molecules C 6 H 12 O 6 by the factor (6 atoms O/1 molecule C 6 H 12 O 6) gives the number of O atoms. Solve: Check: The answer is simply 6 times as large as the answer to part (a). The number of significant figures (three) and the units (atoms O) are correct.
Average Atomic Mass

Let’s say you were sent from a planet far away to find the molar mass of man. Here’s the data you collected: 70% @ 55 lbs 20% @ 135 lbs 10% @ 200 lbs.
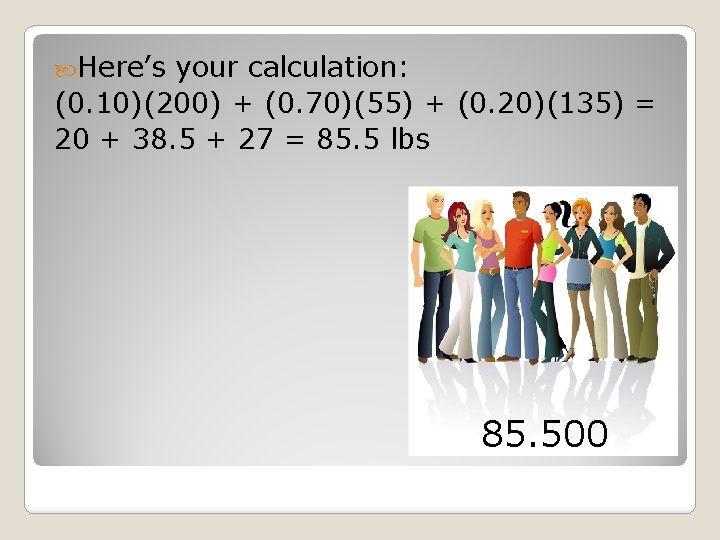
Here’s your calculation: (0. 10)(200) + (0. 70)(55) + (0. 20)(135) = 20 + 38. 5 + 27 = 85. 5 lbs 85. 500
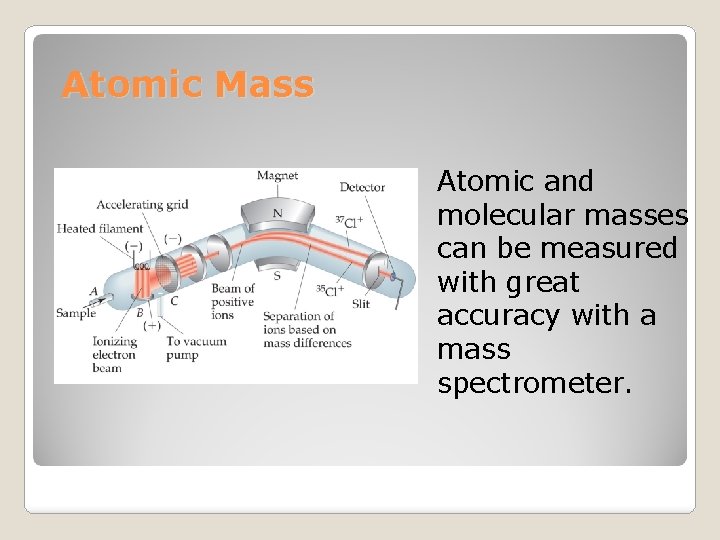
Atomic Mass Atomic and molecular masses can be measured with great accuracy with a mass spectrometer.

Because in the real world we use large amounts of atoms and molecules, we use average masses in calculations. Average mass is calculated from the isotopes of an element weighted by their relative abundances. Average Mass

SAMPLE EXERCISE 2. 4 Calculating the Atomic Weight of an Element from Isotopic Abundances Naturally occurring chlorine is 75. 78% 35 Cl, which has an atomic mass of 34. 969 amu, and 24. 22% 37 Cl, which has an atomic mass of 36. 966 amu. Calculate the average atomic mass (that is, the atomic weight) of chlorine. Solution The average atomic mass is found by multiplying the abundance of each isotope by its atomic mass and summing these products. Because 75. 78% = 0. 7578 and 24. 22% = 0. 2422, we have This answer makes sense: The average atomic mass of Cl is between the masses of the two isotopes and is closer to the value of 35 Cl, which is the more abundant isotope.
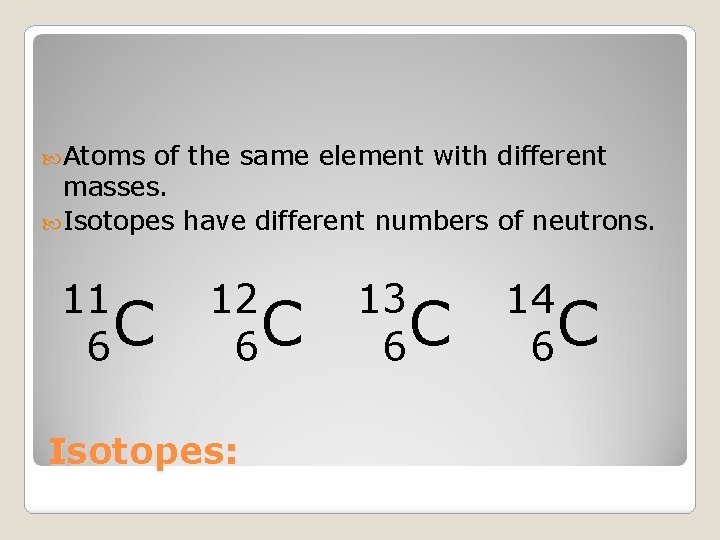
Atoms of the same element with different masses. Isotopes have different numbers of neutrons. 11 C 6 12 C 6 Isotopes: 13 C 6 14 C 6

Resolution of Mixtures Postlab

Results? Why, man, I have gotten lots of results! If I find 10, 000 ways something won't work, I haven't failed. I am not discouraged, because every wrong attempt discarded is often a step forward… --Thomas Alva Edison

�Comment on purity of benzoic acid crystals isolated �Theoretical melting point = 122˚C �Choose your best temperature to calculate % error = │Theoretical – Actual │x 100 Theoretical Comment on precision & accuracy. <10% error is awesome <20% is still good! < 30% is okay… Part A]

Be sure to note difference between silver nitrate test on original sample & on distillate (also if results were not good; suggest why? ) Positive test for chloride ion is the precipitate silver chloride Write double replacement equation: Silver nitrate solution added to aqueous sodium chloride results in a precipitate of silver chloride and aqueous spectator ions of sodium nitrate. Part B] Simple Distillation

Be sure to name type of soda used, density of distillate Calculate % distillate of original sample If density is ≈ 1. 00 g/m. L; then how much of your soda is water? Look for theoretical value… Comment on smell of ester; results of limewater test (if results were not good; suggest why? ) Part C] Fractional Distillation

Error Analysis ◦ % error, sources of error Conclusion ◦ You can separate by parts if it helps. ◦ Accuracy, Precision when applicable Real World Application ◦ Apply to 1 real life use Conclusion Now you have been officially initiated into AP Chemistry…. WELCOME!!

Advice from past students
Hi Mrs. Brunet! Well, I'm just writing to tell you that I realized what a great AP Chem teacher you are. Not that I thought you weren't or anything but after going to my chemistry lectures and stuff, I realized that I actually knew more than I thought. Oh yea. . . I guess I should also tell you that I have to use the lab notebook thing you made us do the first semester. I have to do exact same thing. . . Write the purpose and all the data and stuff. All the memories just came screaming back to me. Anyways I hope you don't have too many brats in your class this year. Take care! Justin Kong 9/20/08

Hi Mrs. Brunet, Do you remember me? i am Esther from last year's ap chem class. Guess What? I am taking general chemistry and the teacher is teaching in a very slow pace. I feel like i know a lot even though I felt lost last year. Right now we are using the Zumdahl new edition and I have my first test on chapters 1 -3 on the 17 th. I'm worried, but I feel like you have prepared me well. Hopefully, I will do well in this class. Oh by the way, I was not there on the last day of school, so I couldn't get my portfolio back. I think it would be a good resource for me. I will not be able to come get it during a school day so I was wondering if you can give it to Janet Sim so I can get it from her on a weekend. Thank You. I will come visit you but the earliest would be my winter break. I miss you. -Esther from class of 08 =] Esther KJ Lee 9/11/08
Mrs. Brunet, Hi! This is Monica from AP Chem two years ago? I hope you remember me =] I couldn't get around to visit Cypress High because my schedule didn't work out well, so I wanted to say hi. I am at USC and still taking general chemistry. (The stuff is mostly review from AP Chem, so that gives me a little bit of break =] ) I wanted to thank you so much for making us go through all the lab reports and lab notebooks. It's making my college chem a lot easier =] haha. . . How are you doing? How are your new classes? =D Monica Kim Regards, Nokyeong Monica Kim nokyeong. kim@usc. edu Monica Kim 12/1/07
- Slides: 23