Unit 2 Review TRUE or FALSE Salt Na
























































- Slides: 57

Unit 2 Review

Ø TRUE or FALSE Salt (Na. Cl) is a pure substance and an element.

ANSWER Ø False… pure substance and compound. l Two elements combined = compound

Ø Which state of matter would take the shape of it’s container and has a fixed/definite volume?

ANSWER Ø Liquid

Ø A popsicle melting on the sidewalk is an example of what type of change?

ANSWER Ø Physical…all phase changes (solid liquid gas) are physical changes.

Ø “A shiny piece of steel rusted from sitting out in the rain. ” Ø Which part of this statement represents a physical property? Which part represents a chemical property?

ANSWER Ø “A shiny piece of steel rusted from sitting out in the rain. ” Ø “SHINY” is physical, describing what it looks like Ø “RUSTING” is chemical, because rust has a different composition than steel.

Ø Matter changing from its liquid state to its gaseous state is called ______.

ANSWER Ø Vaporization l l Boiling Evaporation

Ø What is an ion?

ANSWER Ø An atom with a positive or negative charge. Ø An atom that has lost or gained electrons.

Ø If at ion has a positive charge, did it GAIN electrons or LOSE electrons?

ANSWER Ø LOSE electrons so that there are now more positive protons than there are negative electrons.

Ø What charge would group 7 most likely have?

ANSWER Ø -1

Ø What charge would group 2 most likely have?

ANSWER Ø +2

Ø How many neutrons does Carbon-14 have?

ANSWER Ø 14 is the mass number of that particular isotope of Carbon and the atomic number of Carbon is 6, so 14 -6= 8 neutrons.

Ø TRUE or FALSE To get an isotope, you fluctuate the number of electrons.

ANSWER Ø FALSE. You would fluctuate the number of neutrons to change the mass.

Ø Who discovered protons and how? FIRST AND LAST NAME, BE DETAILED.

ANSWER Ø Ernest Rutherford by shooting alpha particles (positive particles) at a piece of foil. The alpha particles deflected, which showed they were hitting something else that was positive (protons).

Ø Who came up with the first atomic theory? FIRST AND LAST NAME.

ANSWER Ø John Dalton

Ø Who discovered electrons and how? FIRST AND LAST NAME. BE DETAILED.

ANSWER Ø JJ Thomson- cathode ray tube showed electrons are emitted. Negative side of magnet repelled (or positive side attracted) so proved they were negative.

Ø What part of the atom gives it it’s identity?

ANSWER Ø Protons

Ø Would spaghetti be a pure substance or a mixture?

ANSWER Ø Mixture (heterogeneous)

Ø What are the 3 particles of the atom and their respective charges?

ANSWER Ø Proton + Ø Neutron (neutral) Ø Electron -

Ø Why isn’t the mass of strontium-52 on the periodic table reported as 52?

ANSWER Ø The periodic table shows average atomic masses of all isotopes present

Ø How many electrons fit on energy level 1, 2, & 3?

ANSWER Ø 1 st holds 2 Ø 2 nd holds 8 Ø 3 rd holds 8

Ø Who created the atom model where electrons are found on energy levels?

ANSWER Ø Niels Bohr

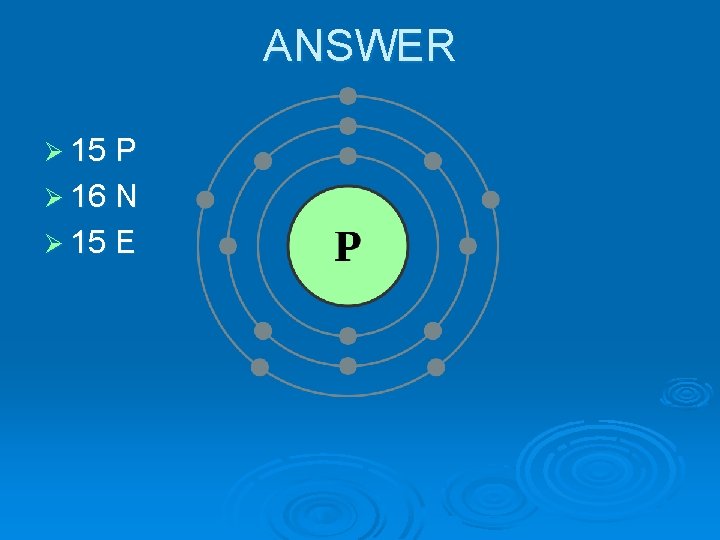
Ø Draw the Bohr diagram for phosphorus.

ANSWER Ø 15 P Ø 16 N Ø 15 E

Ø Write the following info in isotopic notation l l l Protons- 26 Neutrons- 31 Electrons- 23

ANSWER Ø 57 Fe 3+ 26

Atomic Number Ø Mass Number Ø Neutrons Ø Electrons Ø

ANSWER Ø Atomic Number- 16 Ø Mass Number- 33 Ø Neutrons- 17 Ø Electrons -18

Ø Who discovered the neutron? FIRST AND LAST NAME.

ANSWER Ø James Chadwick

Ø Protons-20 Ø Electrons-20 Ø Neutrons-22 Ø Isotope name?

ANSWER Ø Calcium-42

Ø Protons-20 Ø Electrons-20 Ø Neutrons-22 Ø Give the correct notation.

ANSWER

Ø What is the name of the element if the only information you have is there are 35 protons?

ANSWER Ø Bromine

Ø List techniques for physically separating a mixture (think about our lab)

ANSWER Ø Filtering Ø Using a magnet Ø Boiling Ø Dissolving