UNIT 2 PROPERTIES OF MATTER NONCHARACTERISTIC PROPERTIES OF




















- Slides: 46

UNIT 2: PROPERTIES OF MATTER - NON-CHARACTERISTIC PROPERTIES OF MATTER 1. 2. 3. 4. 5. - STATES OF MATTER MASS VOLUME TEMPERATURE ACIDS & BASES CHARACTERISTIC PROPERTIES OF MATTER 1. 2. THE MELTING POINT THE BOILING POINT

NON-CHARACTERISTIC PROPERTIES �NON-CHARACTERISTIC PROPERTY: A property that may be common to several substances or individuals; it does not allow to precisely identify a substance or individual. For Example: One student may have brown hair in the class. This property alone is not enough to recognize the individual because several classmates may have brown hair.

NON-CHARACTERISTIC PROPERTIES �Non-Characteristic Properties include: 1 - The States of Matter 2 - Mass 3 - Volume 4 - Temperature 5 - Acids and bases

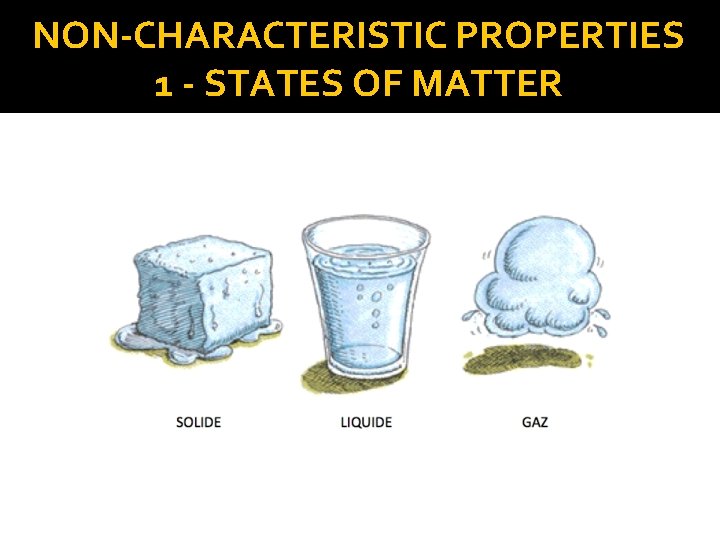
NON-CHARACTERISTIC PROPERTIES 1 - STATES OF MATTER � What is MATTER? Matter - Anything that has mass and occupies space. � The three main states of matter: 1 – Solid 2 – Liquid 3 – Gas � https: //www. khanacademy. org/science/chemistry/states-of-matter-and-intermolecular-forces/states-of-matter/v/states-of-matter

NON-CHARACTERISTIC PROPERTIES 1 - STATES OF MATTER

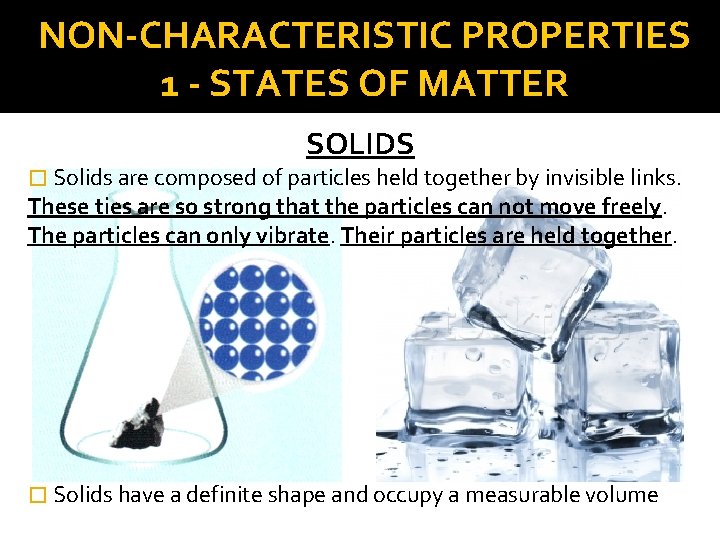
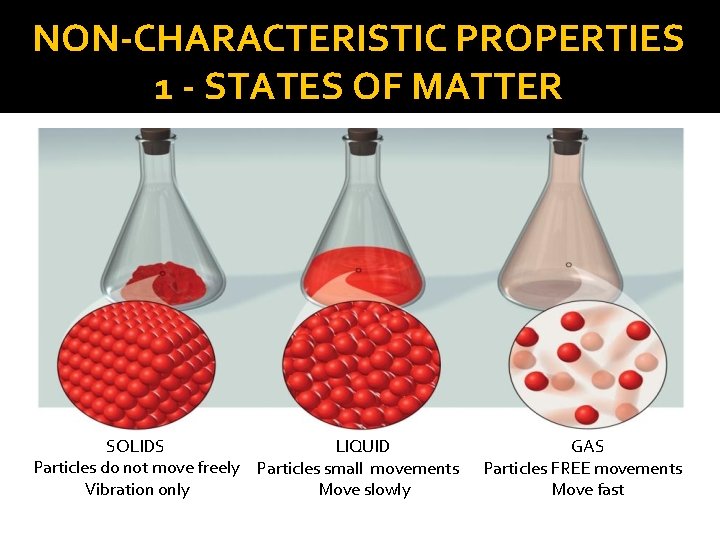
NON-CHARACTERISTIC PROPERTIES 1 - STATES OF MATTER SOLIDS � Solids are composed of particles held together by invisible links. These ties are so strong that the particles can not move freely. The particles can only vibrate. Their particles are held together. � Solids have a definite shape and occupy a measurable volume

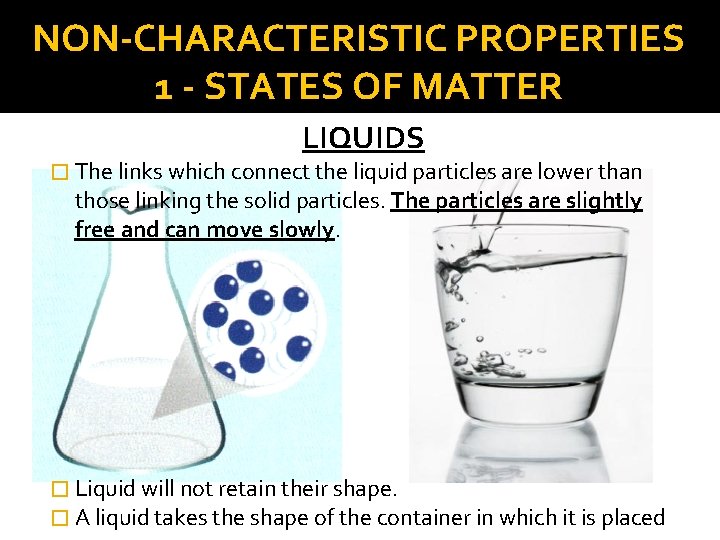
NON-CHARACTERISTIC PROPERTIES 1 - STATES OF MATTER LIQUIDS � The links which connect the liquid particles are lower than those linking the solid particles. The particles are slightly free and can move slowly. � Liquid will not retain their shape. � A liquid takes the shape of the container in which it is placed

NON-CHARACTERISTIC PROPERTIES 1 - STATES OF MATTER GAS � The links between the particles of gas are even lower than those linking the particles of a liquid. Particles can move much more freely than those of solids and liquids - There are large gaps between the particles � As their particles can move very freely, gas particles can circulate in all directions. � A gas spreads to fill the space of a container or a part.

NON-CHARACTERISTIC PROPERTIES 1 - STATES OF MATTER SOLIDS LIQUID Particles do not move freely Particles small movements Vibration only Move slowly GAS Particles FREE movements Move fast

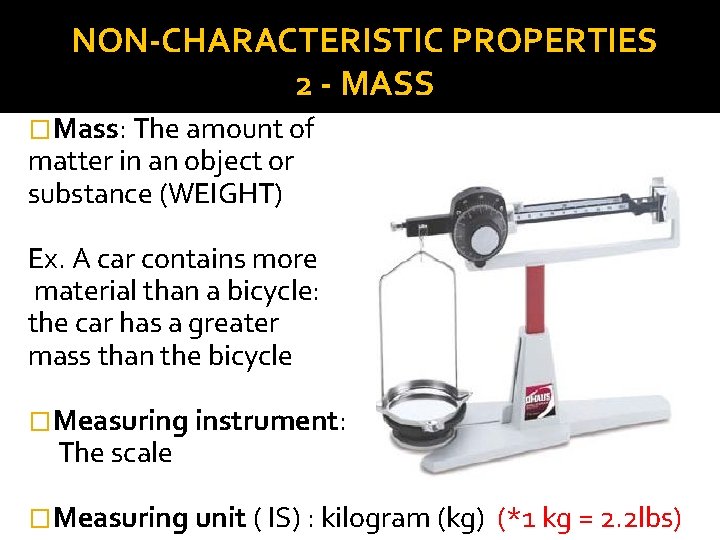
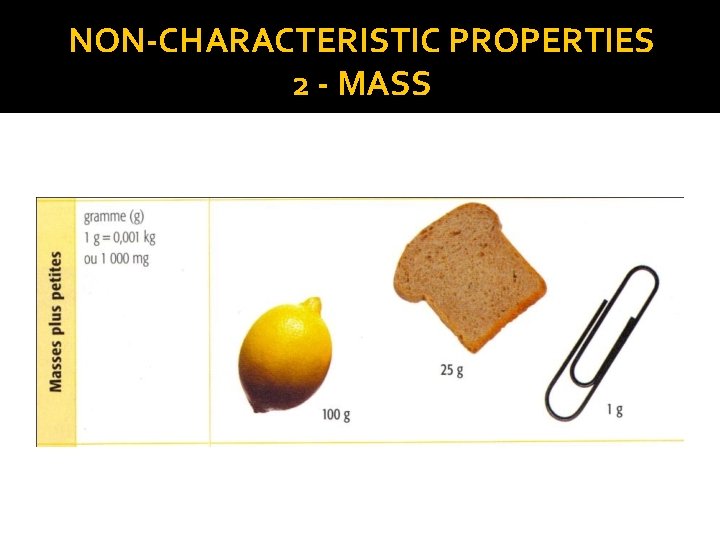
NON-CHARACTERISTIC PROPERTIES 2 - MASS �Mass: The amount of matter in an object or substance (WEIGHT) Ex. A car contains more material than a bicycle: the car has a greater mass than the bicycle �Measuring instrument: The scale �Measuring unit ( IS) : kilogram (kg) (*1 kg = 2. 2 lbs)

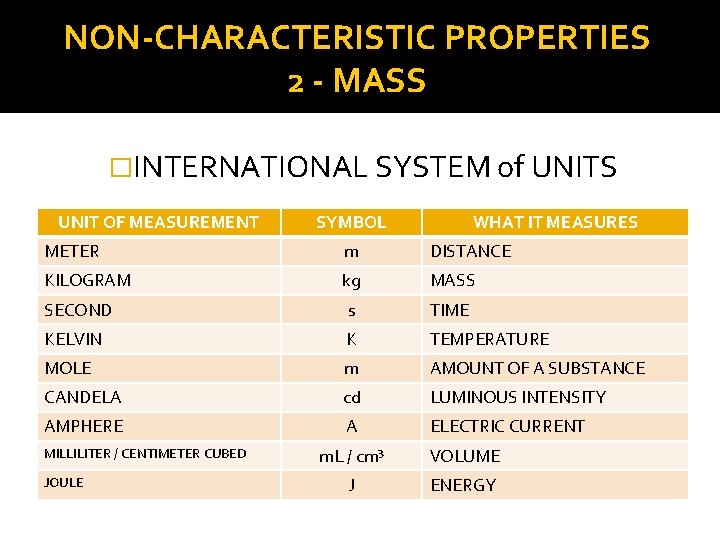
NON-CHARACTERISTIC PROPERTIES 2 - MASS �INTERNATIONAL SYSTEM of UNITS UNIT OF MEASUREMENT SYMBOL WHAT IT MEASURES METER m DISTANCE KILOGRAM kg MASS SECOND s TIME KELVIN K TEMPERATURE MOLE m AMOUNT OF A SUBSTANCE CANDELA cd LUMINOUS INTENSITY AMPHERE A ELECTRIC CURRENT MILLILITER / CENTIMETER CUBED JOULE m. L / cm³ VOLUME J ENERGY

NON-CHARACTERISTIC PROPERTIES 2 - MASS

NON-CHARACTERISTIC PROPERTIES 2 - MASS

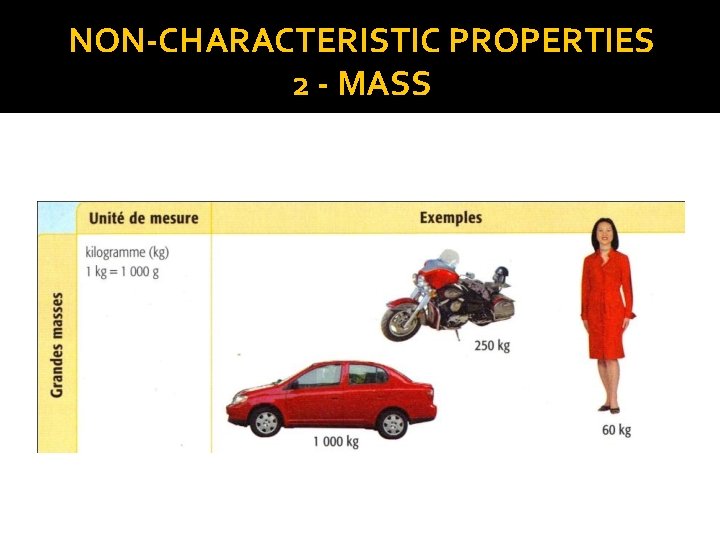
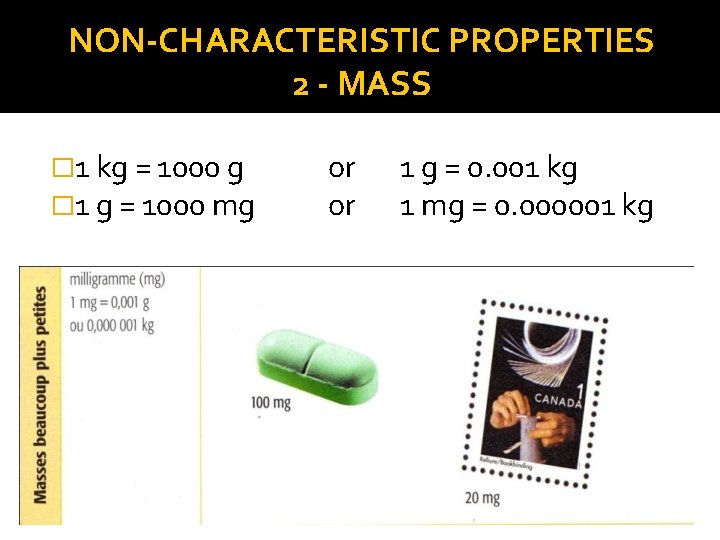
NON-CHARACTERISTIC PROPERTIES 2 - MASS � 1 kg = 1000 g � 1 g = 1000 mg or or 1 g = 0. 001 kg 1 mg = 0. 000001 kg

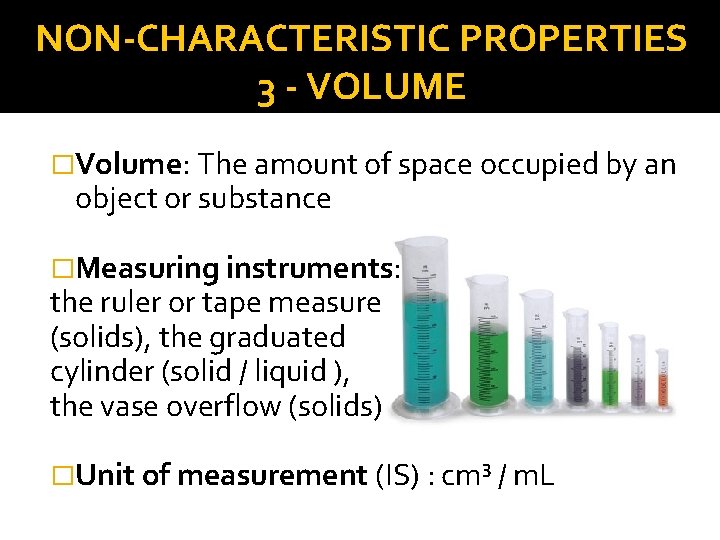
NON-CHARACTERISTIC PROPERTIES 3 - VOLUME �Volume: The amount of space occupied by an object or substance �Measuring instruments: the ruler or tape measure (solids), the graduated cylinder (solid / liquid ), the vase overflow (solids) �Unit of measurement (IS) : cm³ / m. L

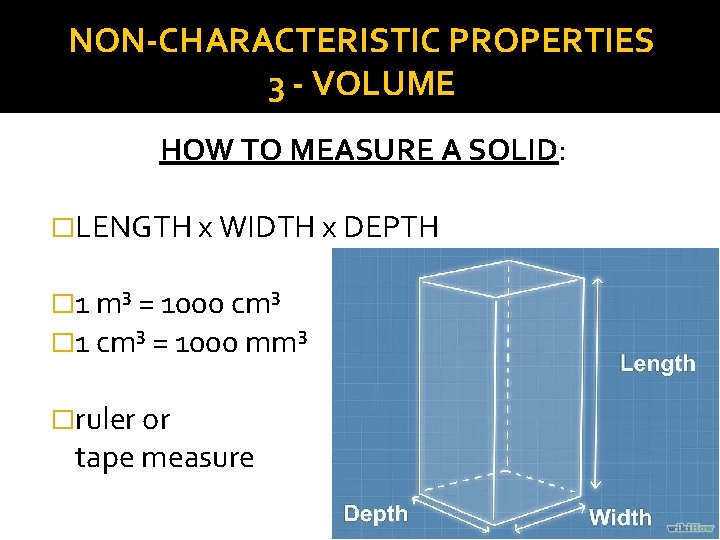
NON-CHARACTERISTIC PROPERTIES 3 - VOLUME HOW TO MEASURE A SOLID: �LENGTH x WIDTH x DEPTH � 1 m³ = 1000 cm³ � 1 cm³ = 1000 mm³ �ruler or tape measure

NON-CHARACTERISTIC PROPERTIES 3 - VOLUME HOW TO MEASURE A SOLID / LIQUID: �PLACE LIQUID IN THE MEASURING CUP �PLACE SOLID IN THE GRADUATED CYLINDER AND ADD LIQUID � 1 m. L = 1 cm³

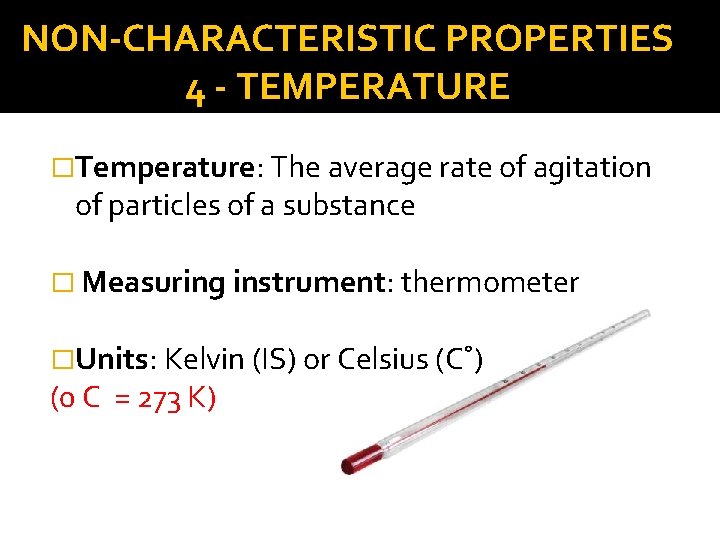
NON-CHARACTERISTIC PROPERTIES 4 - TEMPERATURE �Temperature: The average rate of agitation of particles of a substance � Measuring instrument: thermometer �Units: Kelvin (IS) or Celsius (C˚) (0 C = 273 K)

NON-CHARACTERISTIC PROPERTIES 4 - TEMPERATURE �In a glass of water (liquid), the particles move more easily than the particles in an ice cube (solid) where the particles are almost motionless. This is because the speed of the glass particles of the liquid is warmer than the ice.

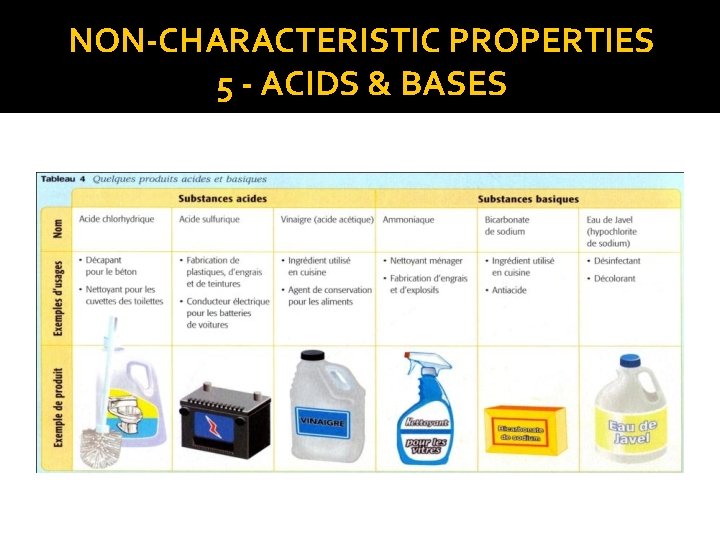
NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �The sour and spicy tastes are properties of acidic substances �The bitter taste is a property of basic substances

NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES ACIDS BASES

NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES

NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �Your own body produces acids and strong bases �The stomach secretes hydrochloric acid, which attacks the food to extract nutrients � The pancreas secretes bicarbonate (base) to neutralize the effect of acid when the food is in the small intestine

NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �Some insects ( bees , wasps and some ants ) produce a highly acidic venom to defend themselves �When one gets stung or bitten , the venom of the acid reacts with the water in skin cells. �You can use baking soda in water (base) to relieve the burning or the sting

NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �Some plants, such as poison ivy, produce a basic substance for defense �When the water in the skin comes into contact with this substance, we feel a violent irritation. �A slightly acidic substance may be used (vinegar or lemon juice) to relieve the itching.

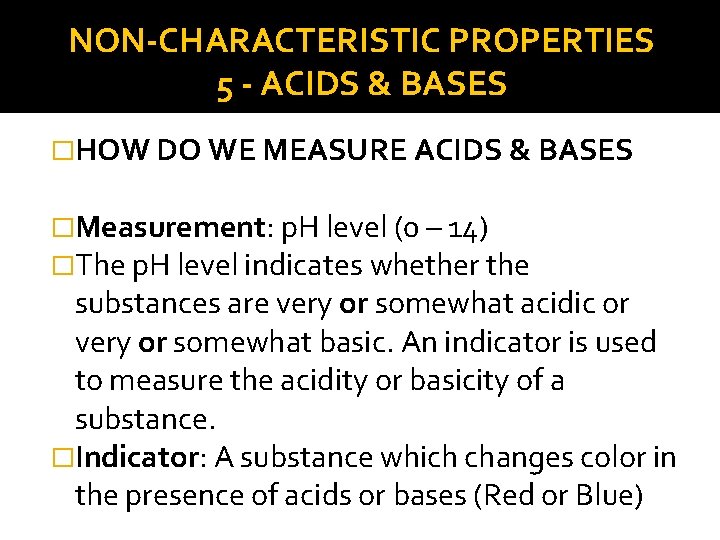
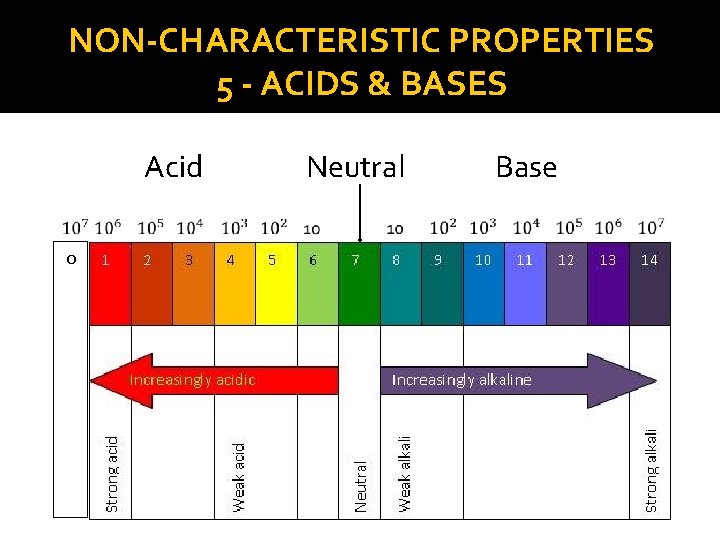
NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �HOW DO WE MEASURE ACIDS & BASES �Measurement: p. H level (0 – 14) �The p. H level indicates whether the substances are very or somewhat acidic or very or somewhat basic. An indicator is used to measure the acidity or basicity of a substance. �Indicator: A substance which changes color in the presence of acids or bases (Red or Blue)

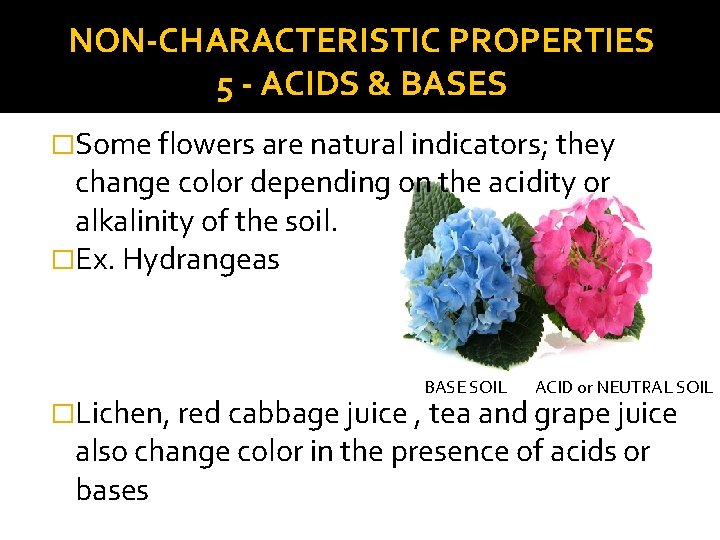
NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �Some flowers are natural indicators; they change color depending on the acidity or alkalinity of the soil. �Ex. Hydrangeas BASE SOIL ACID or NEUTRAL SOIL �Lichen, red cabbage juice , tea and grape juice also change color in the presence of acids or bases

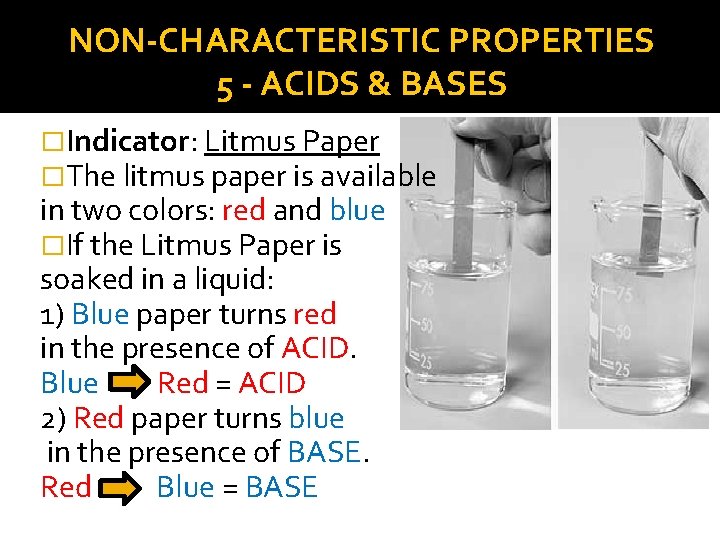
NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �Indicator: Litmus Paper �The litmus paper is available in two colors: red and blue �If the Litmus Paper is soaked in a liquid: 1) Blue paper turns red in the presence of ACID. Blue Red = ACID 2) Red paper turns blue in the presence of BASE. Red Blue = BASE

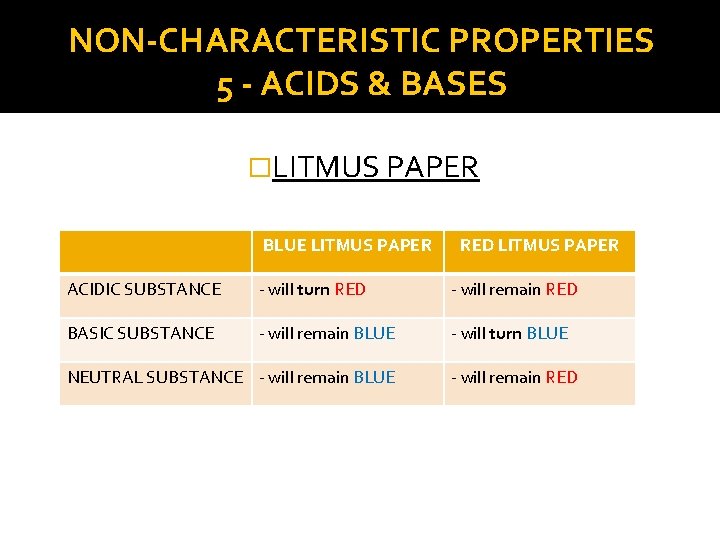
NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �LITMUS PAPER BLUE LITMUS PAPER RED LITMUS PAPER ACIDIC SUBSTANCE - will turn RED - will remain RED BASIC SUBSTANCE - will remain BLUE - will turn BLUE NEUTRAL SUBSTANCE - will remain BLUE - will remain RED

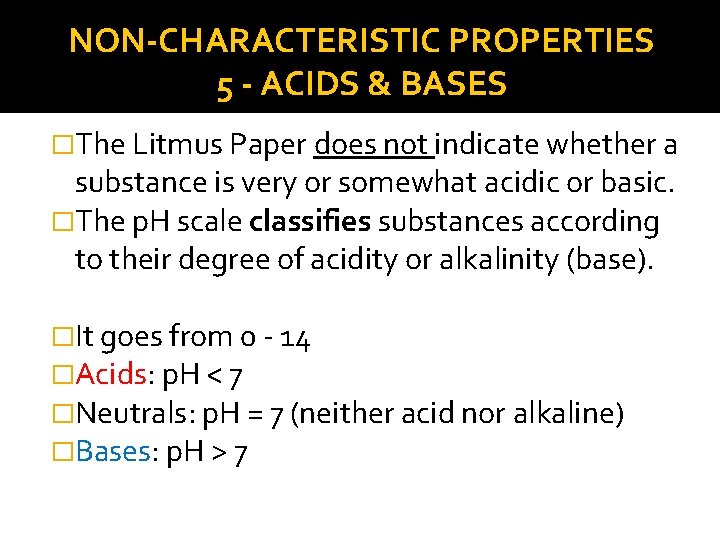
NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �The Litmus Paper does not indicate whether a substance is very or somewhat acidic or basic. �The p. H scale classifies substances according to their degree of acidity or alkalinity (base). �It goes from 0 - 14 �Acids: p. H < 7 �Neutrals: p. H = 7 (neither acid nor alkaline) �Bases: p. H > 7

NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES

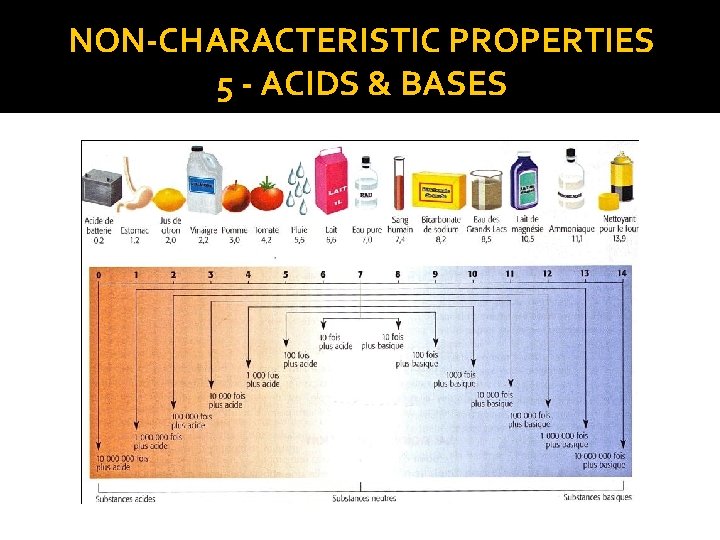
NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �Each degree of the p. H scale represents a factor of 10 (multiplied by 10) �Ex. The p. H of an apple = 3 and the p. H of a lemon = 2. The Lemon is 10 times more acidic than the apple.

NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES Acid 0 Neutral Base

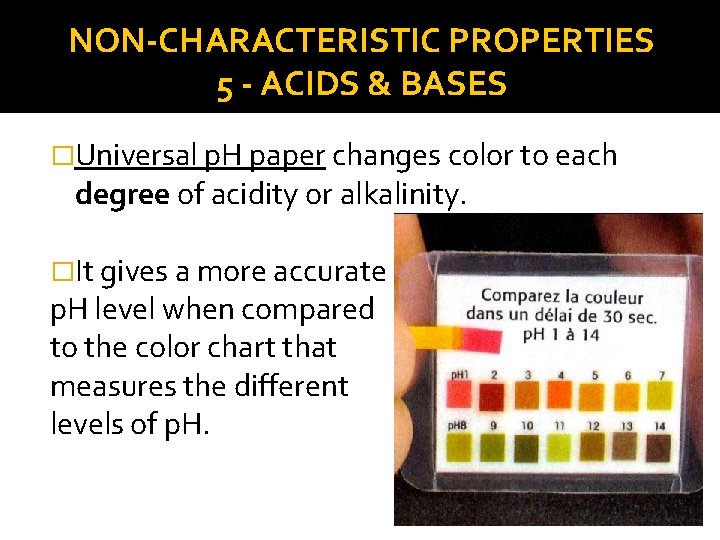
NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �Universal p. H paper changes color to each degree of acidity or alkalinity. �It gives a more accurate p. H level when compared to the color chart that measures the different levels of p. H.

NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �The p. H meter is an electronic device that directly gives the value of p. H of a substance. �It uses the ability of liquid substances to conduct an electrical current. �The more substance is acidic or basic , the better it conducts electricity.

NON-CHARACTERISTIC PROPERTIES 5 - ACIDS & BASES �MEASURING DEVICES � 1) LITMUS PAPER – only indicates whether or not the substance is an ACID or BASE � 2) UNIVERSAL p. H PAPER – indicates the level of ACIDITY or ALKALINITY � 3) p. H METER – digital reading pinpointing levels of ACIDITY or ALKALINITY

QUALITATIVE vs. QUANTITATIVE �Properties help to identify an unknown foreign matter. �All material has characteristics and non- characteristic properties. �Properties can also categorized another way: QUALITATIVE and QUANTITATIVE.

QUALITATIVE vs. QUANTITATIVE �The majority of properties can be measured with an instrument. Ex - scale, graduated cylinder, thermometer �We call these properties QUANTITATIVE. These properties are ALWAYS expressed with a number and a unit. 1) Mass 2) Volume 3) Temperature

QUALITATIVE vs. QUANTITATIVE �In addition, there are other properties which are not measurable. �They can only be observed by our senses. � Because these observations have no number or unit of measurement associated with it, we call these properties QUALITATIVE. 1) Color 2) Conductivity 3) Smell 4) Clarity

QUALITATIVE vs. QUANTITATIVE �Conductivity - The material is able to transfer electricity. - Water and Metal (Yes) - Rubber (No) �Smell - What smell does matter have? - Compare it to smells you already know. Ex - vinegar, alcohol, fruity, no smell etc. - You can also stated that you do not know that smell (Unknown)

QUALITATIVE vs. QUANTITATIVE �Color - can be used to describe certain material and their reactions in different lights. �Clarity - Is the material transparent or not? Ex - Transparent: What you can see if it's in a liquid (sediment), gas (cloud), solid (different colors)

CHARACTERISTIC PROPERTIES �Characteristic property: A property that allows to accurately identify a substance or an individual. For Example: A person’s fingerprint can be used to specifically identify a person because no TWO fingerprints are the same.

CHARACTERISTIC PROPERTIES �Characteristic properties of Matter include: 1) 2) The melting point (solid to liquid) The boiling point (liquid to gas) �The temperatures at which a substance changes state is an example of a characteristic property.

CHARACTERISTIC PROPERTIES MELTING POINT �MELTING – The transition from the solid to the liquid state �Melting point: The temperature at which the melting is observed (Fusion Point) �Water = 0º C (32º F or 273º K)

CHARACTERISTIC PROPERTIES BOILING POINT �BOILING – The transition from liquid to gaseous state �Boiling point: The temperature at which boiling is observed. (Evaporation) �Water = 100º C (180º F or 373º K)

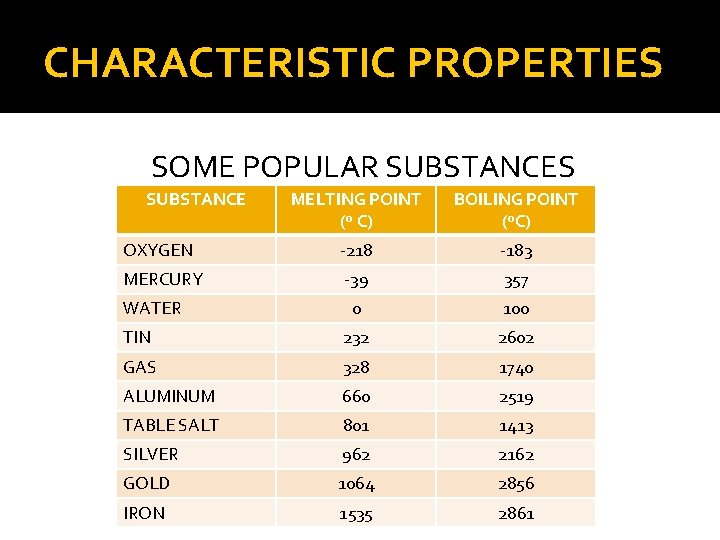
CHARACTERISTIC PROPERTIES SOME POPULAR SUBSTANCES SUBSTANCE MELTING POINT (º C) BOILING POINT (ºC) OXYGEN -218 -183 MERCURY -39 357 0 100 TIN 232 2602 GAS 328 1740 ALUMINUM 660 2519 TABLE SALT 801 1413 SILVER 962 2162 GOLD 1064 2856 IRON 1535 2861 WATER