Unit 2 Part 3 Notes Macromolecules AP Biology

Unit 2, Part 3 Notes: Macromolecules AP Biology

Macromolecules • Macromolecules = large, organic (carboncontaining) molecules • Carbohydrates, Lipids, Proteins, and Nucleic Acids • Always contain carbon, hydrogen, and oxygen • Sometimes contain nitrogen, phosphorus, and sulfur • 6 most common elements in living things = CHNOPS
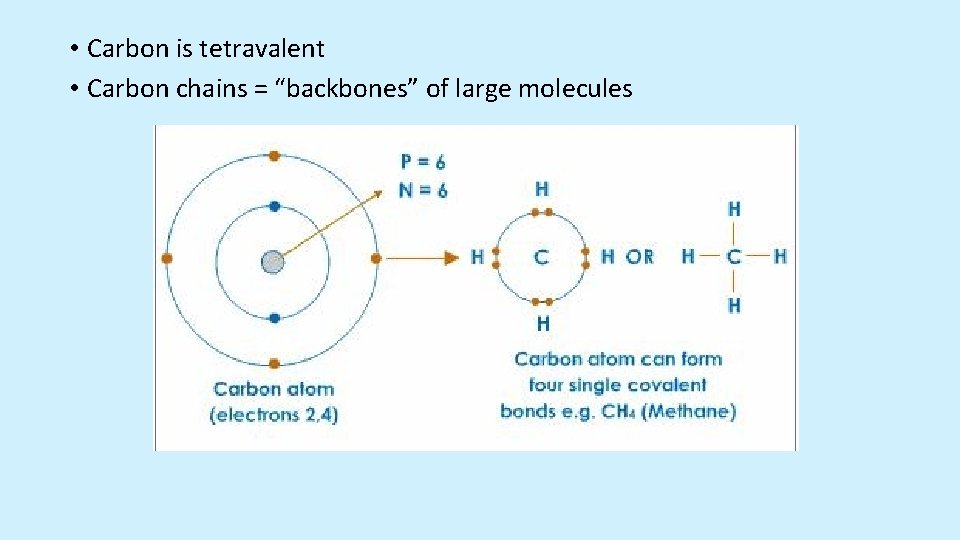
• Carbon is tetravalent • Carbon chains = “backbones” of large molecules
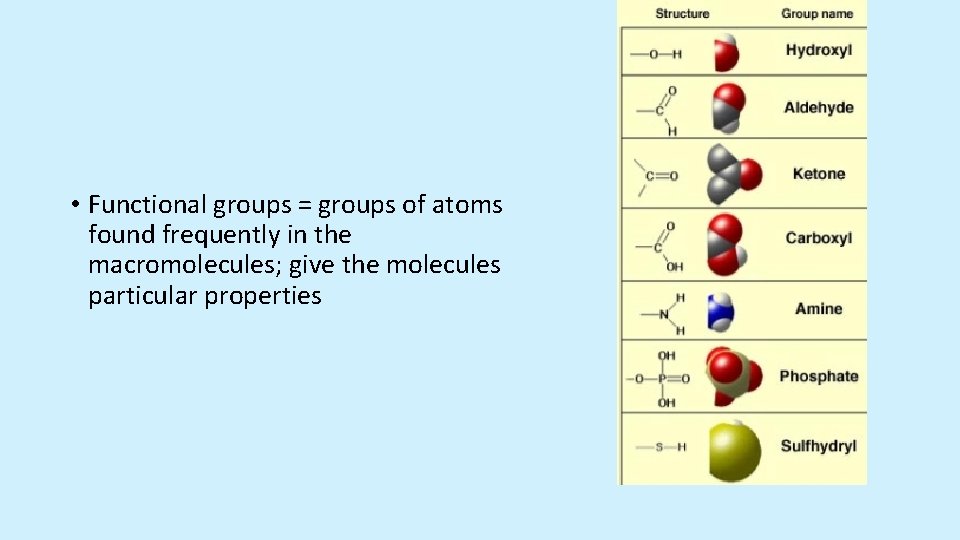
• Functional groups = groups of atoms found frequently in the macromolecules; give the molecules particular properties
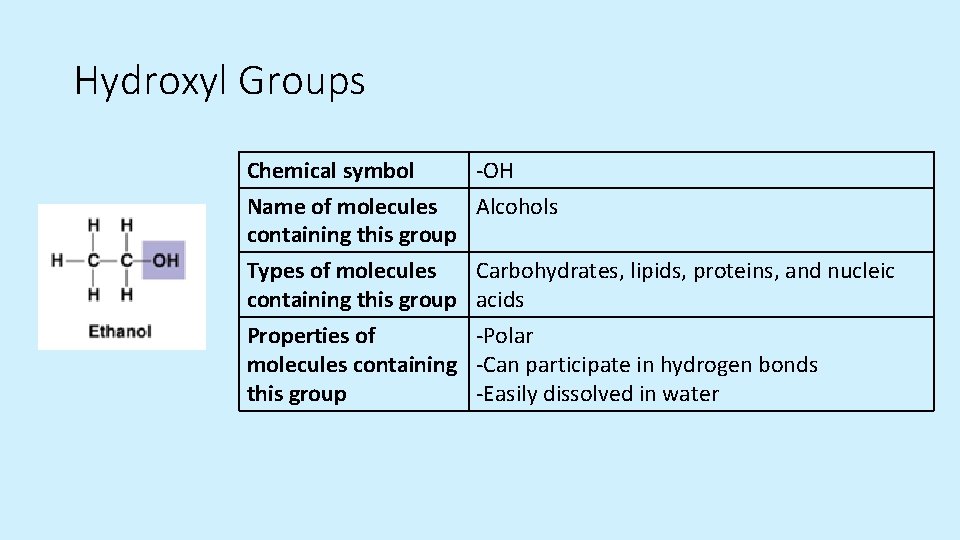
Hydroxyl Groups Chemical symbol Name of molecules containing this group Types of molecules containing this group -OH Alcohols Carbohydrates, lipids, proteins, and nucleic acids Properties of -Polar molecules containing -Can participate in hydrogen bonds this group -Easily dissolved in water
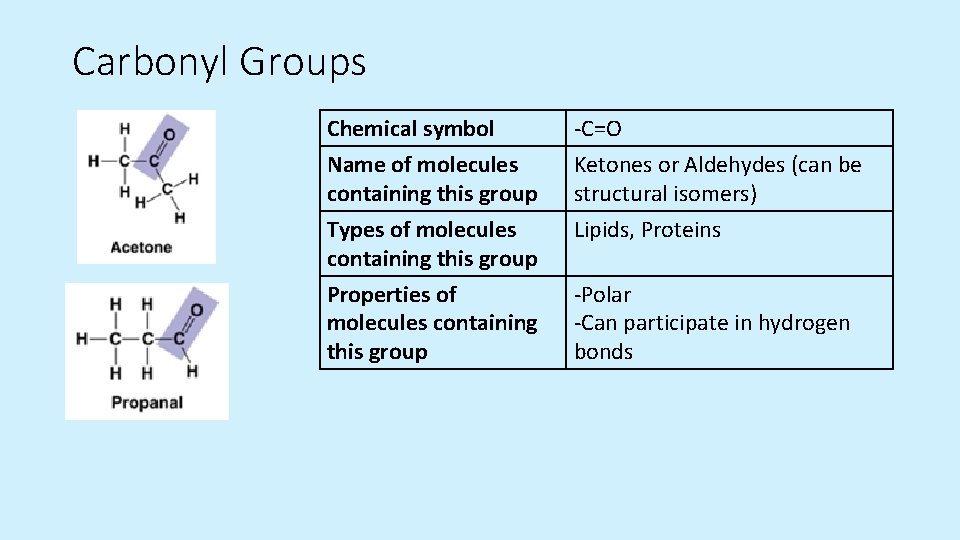
Carbonyl Groups Chemical symbol Name of molecules containing this group -C=O Ketones or Aldehydes (can be structural isomers) Types of molecules containing this group Properties of molecules containing this group Lipids, Proteins -Polar -Can participate in hydrogen bonds
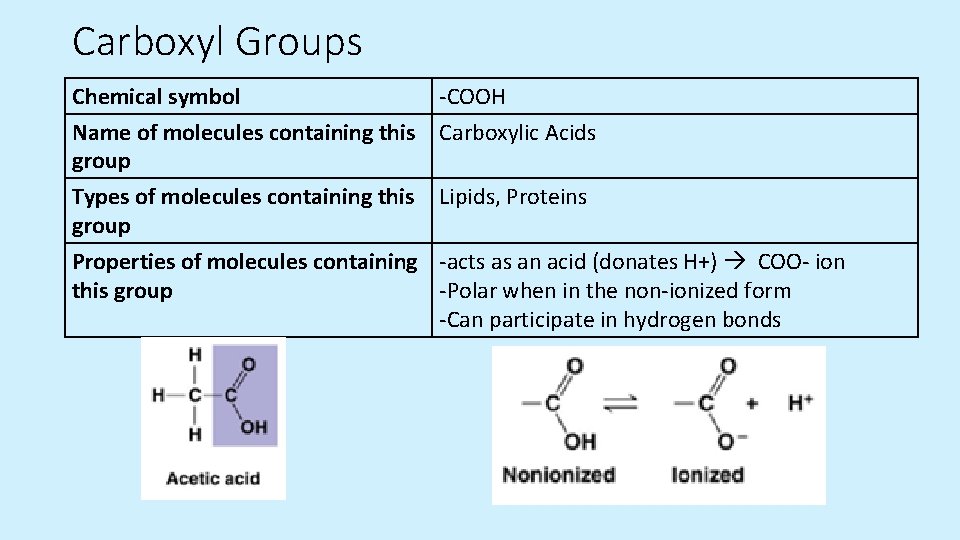
Carboxyl Groups Chemical symbol -COOH Name of molecules containing this Carboxylic Acids group Types of molecules containing this Lipids, Proteins group Properties of molecules containing -acts as an acid (donates H+) COO- ion this group -Polar when in the non-ionized form -Can participate in hydrogen bonds
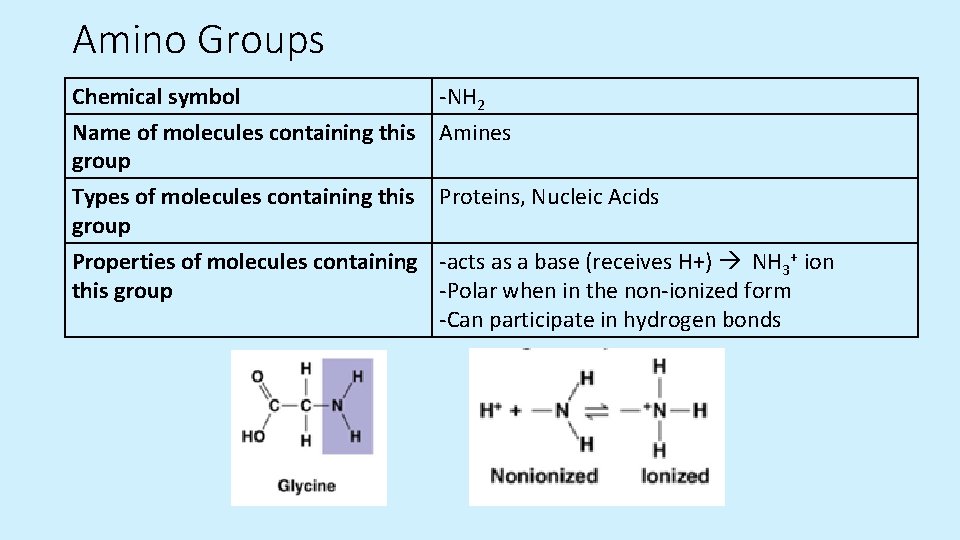
Amino Groups Chemical symbol -NH 2 Name of molecules containing this Amines group Types of molecules containing this Proteins, Nucleic Acids group Properties of molecules containing -acts as a base (receives H+) NH 3+ ion this group -Polar when in the non-ionized form -Can participate in hydrogen bonds
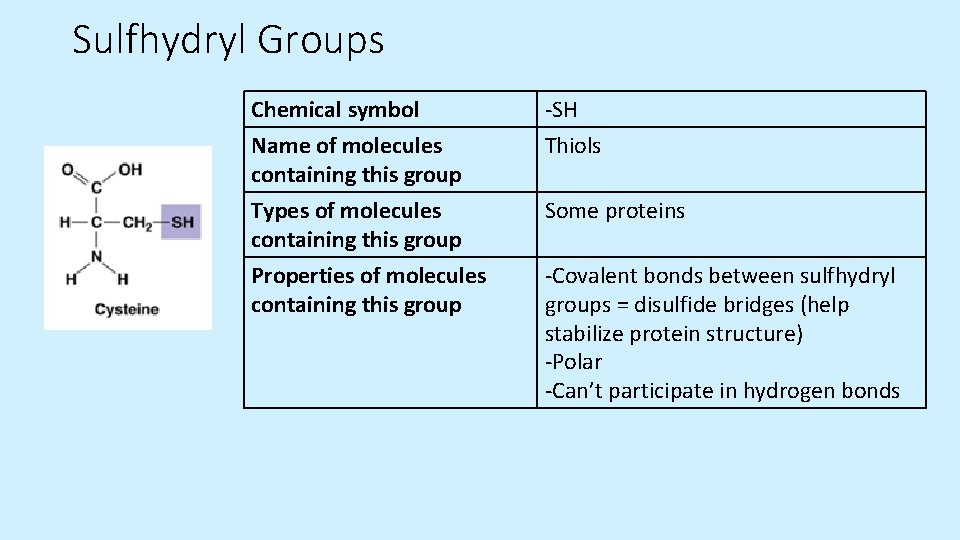
Sulfhydryl Groups Chemical symbol Name of molecules containing this group -SH Thiols Types of molecules containing this group Properties of molecules containing this group Some proteins -Covalent bonds between sulfhydryl groups = disulfide bridges (help stabilize protein structure) -Polar -Can’t participate in hydrogen bonds
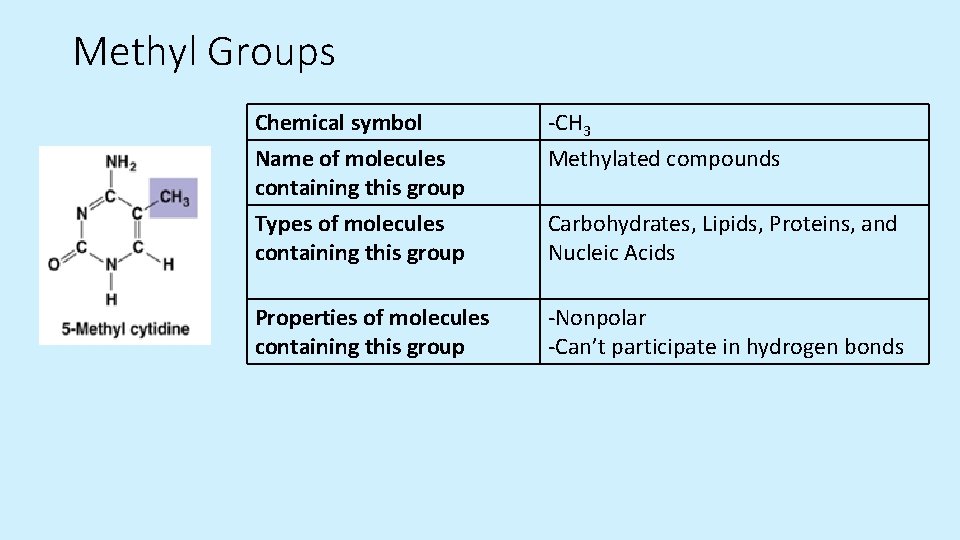
Methyl Groups Chemical symbol Name of molecules containing this group -CH 3 Methylated compounds Types of molecules containing this group Carbohydrates, Lipids, Proteins, and Nucleic Acids Properties of molecules containing this group -Nonpolar -Can’t participate in hydrogen bonds
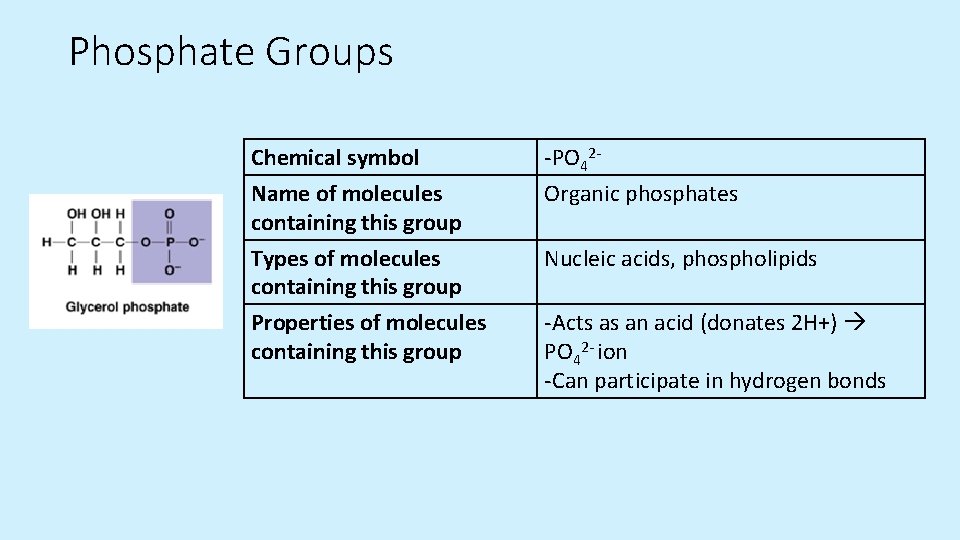
Phosphate Groups Chemical symbol Name of molecules containing this group -PO 42 Organic phosphates Types of molecules containing this group Nucleic acids, phospholipids Properties of molecules containing this group -Acts as an acid (donates 2 H+) PO 42 - ion -Can participate in hydrogen bonds

Index Cards – use page 40 in the book to help • On the front: a drawing of just the functional group • On the back: • • The name of the functional group Properties (polar, etc) What macromolecules it’s found in A drawing of actual example of a full molecule that has this functional group
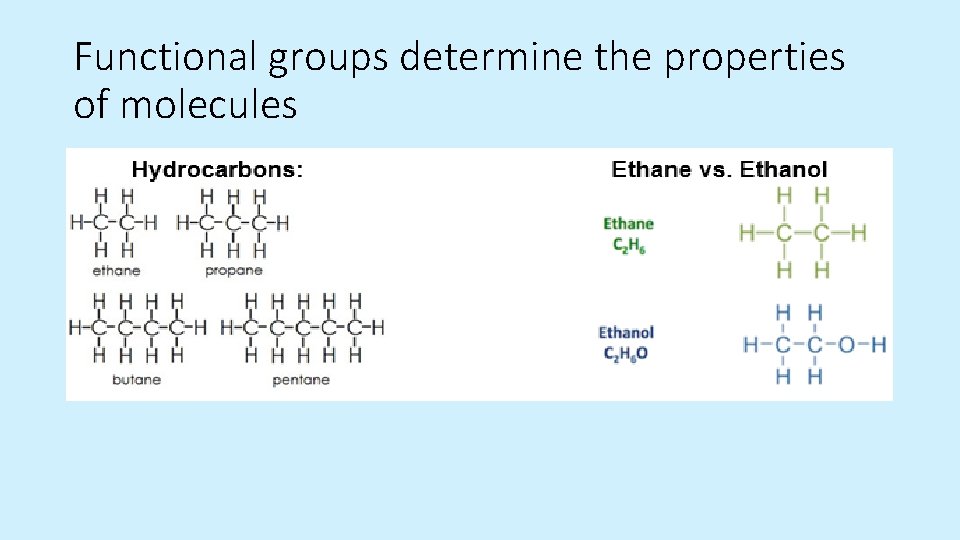
Functional groups determine the properties of molecules
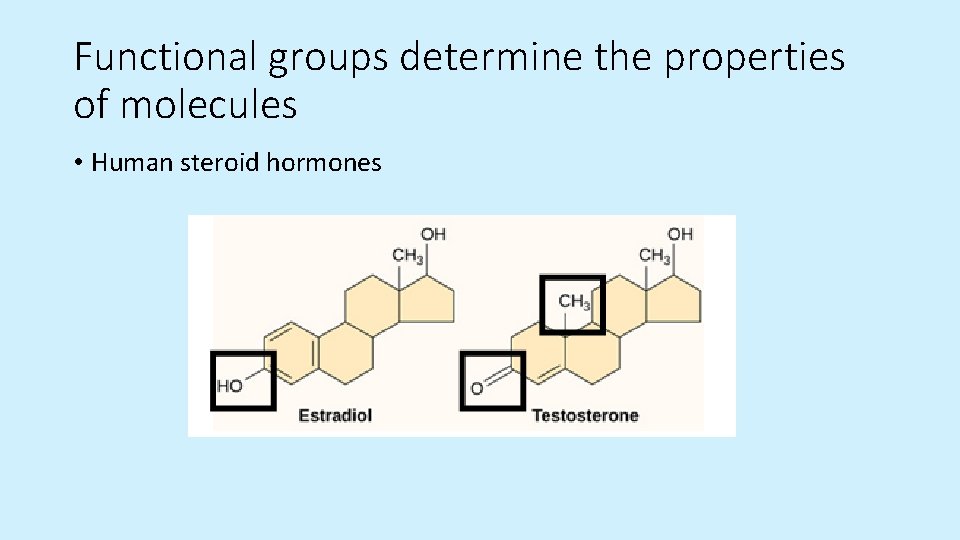
Functional groups determine the properties of molecules • Human steroid hormones
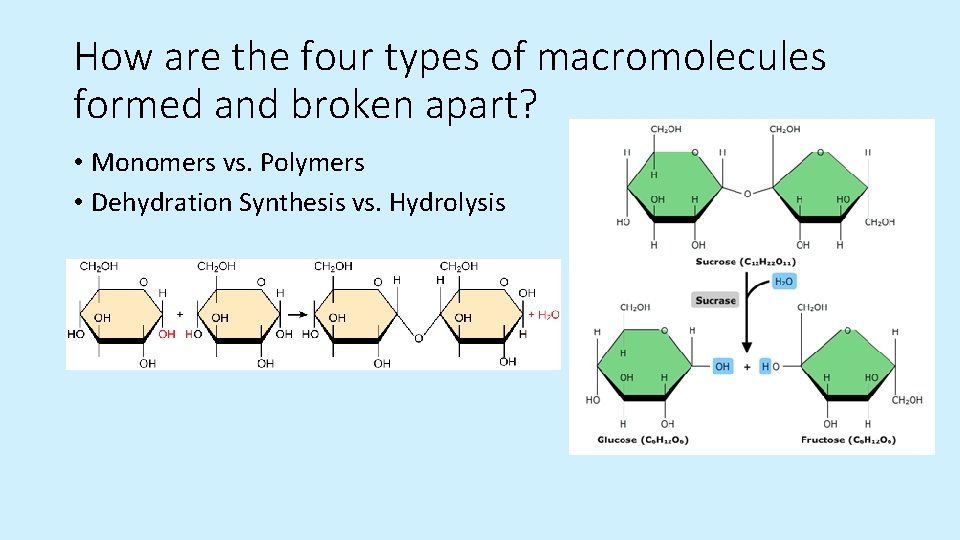
How are the four types of macromolecules formed and broken apart? • Monomers vs. Polymers • Dehydration Synthesis vs. Hydrolysis
Carbohydrates • Elements: C, H, O (1: 2: 1 ratio) • Functions: Short-term energy storage, structure in plant and fungus cell walls, structure in exoskeletons of insects
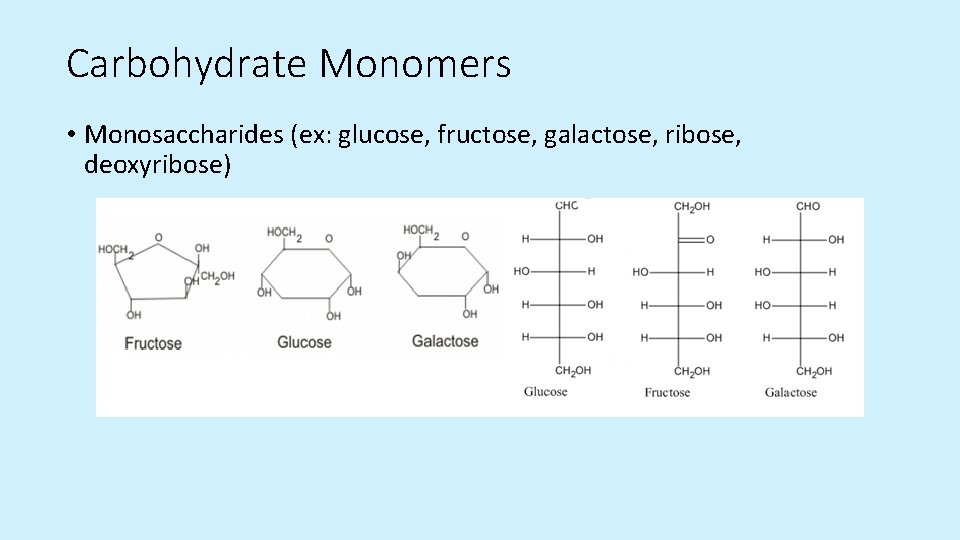
Carbohydrate Monomers • Monosaccharides (ex: glucose, fructose, galactose, ribose, deoxyribose)
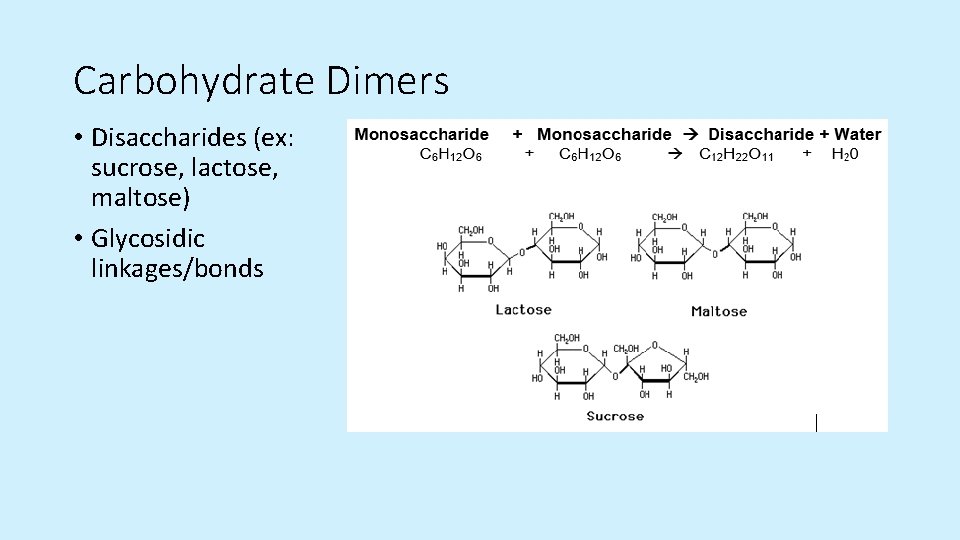
Carbohydrate Dimers • Disaccharides (ex: sucrose, lactose, maltose) • Glycosidic linkages/bonds
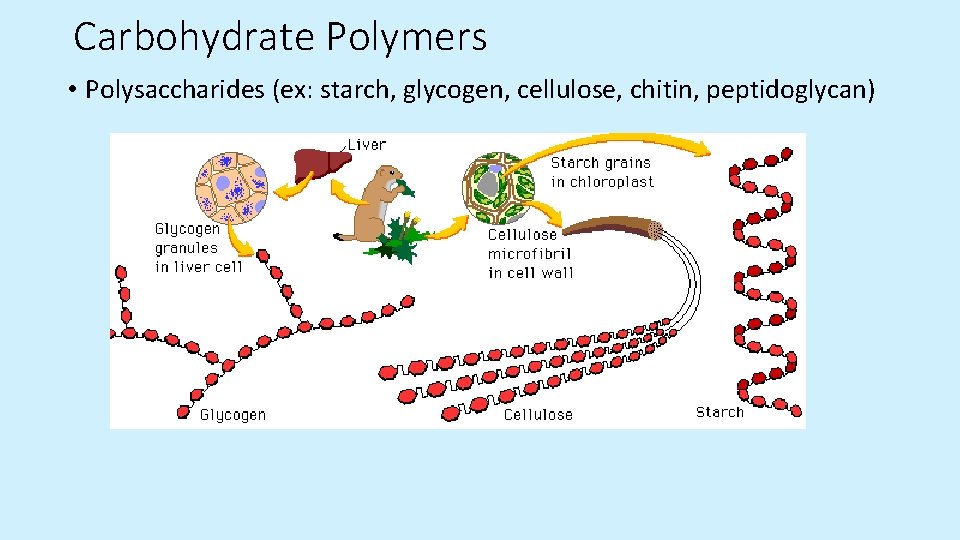
Carbohydrate Polymers • Polysaccharides (ex: starch, glycogen, cellulose, chitin, peptidoglycan)
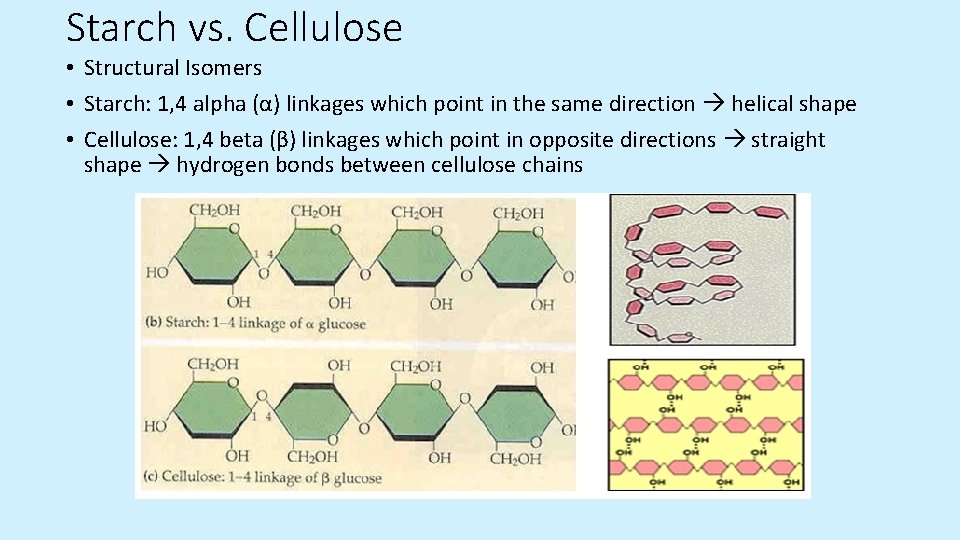
Starch vs. Cellulose • Structural Isomers • Starch: 1, 4 alpha (α) linkages which point in the same direction helical shape • Cellulose: 1, 4 beta (β) linkages which point in opposite directions straight shape hydrogen bonds between cellulose chains

Adaptations for Cellulose Digestion • Ruminants • Caecophores • Pandas? and new research

Lipids • Elements: C, H, O (only a few O’s) • Functions: long-term energy storage, protective coatings (ex: cell membranes), and insulation (ex: whale blubber)
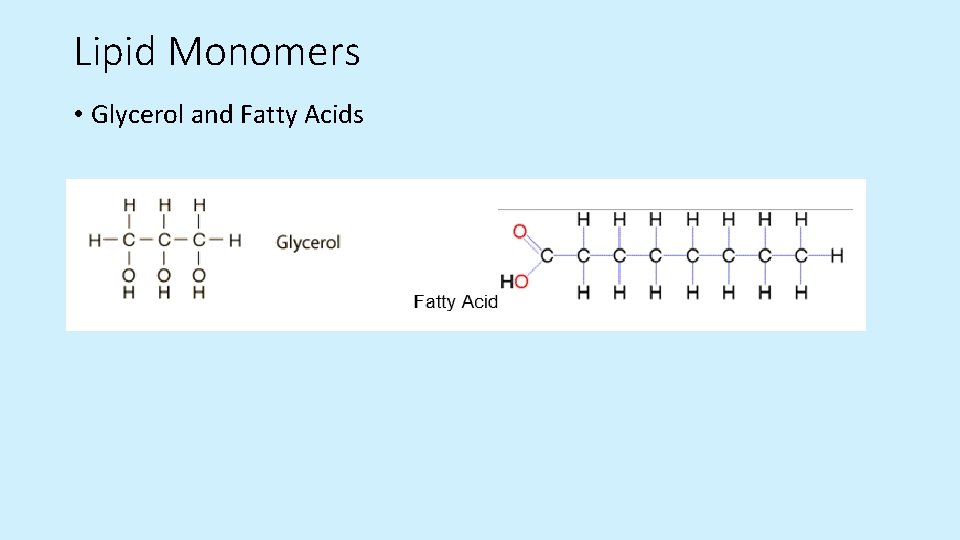
Lipid Monomers • Glycerol and Fatty Acids

Lipid Polymers • Fats (triglycerides), oils, waxes, phospholipids, steroids
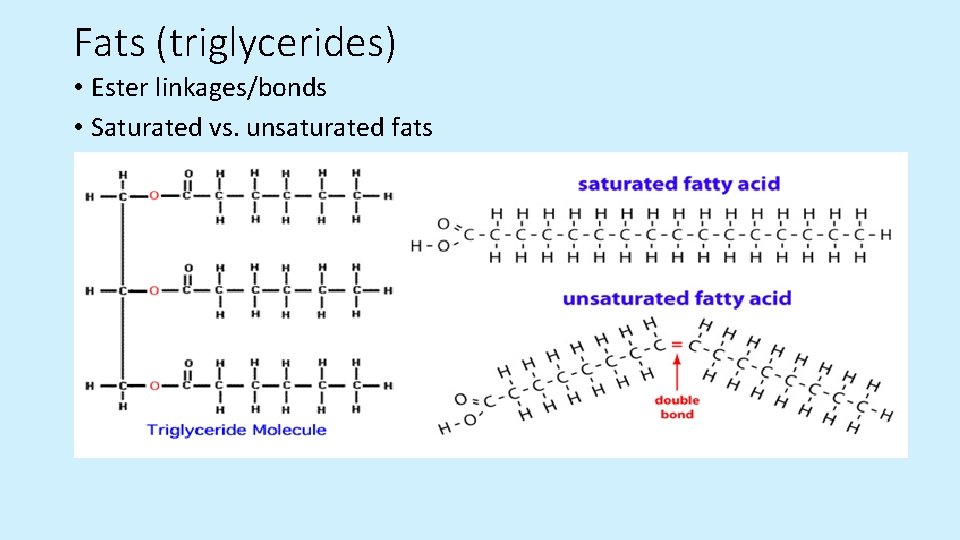
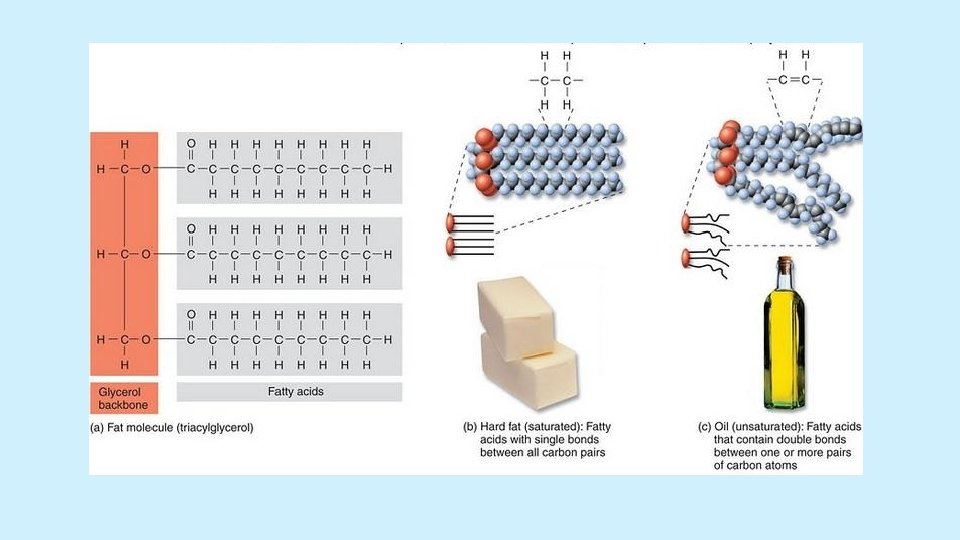
Fats (triglycerides) • Ester linkages/bonds • Saturated vs. unsaturated fats
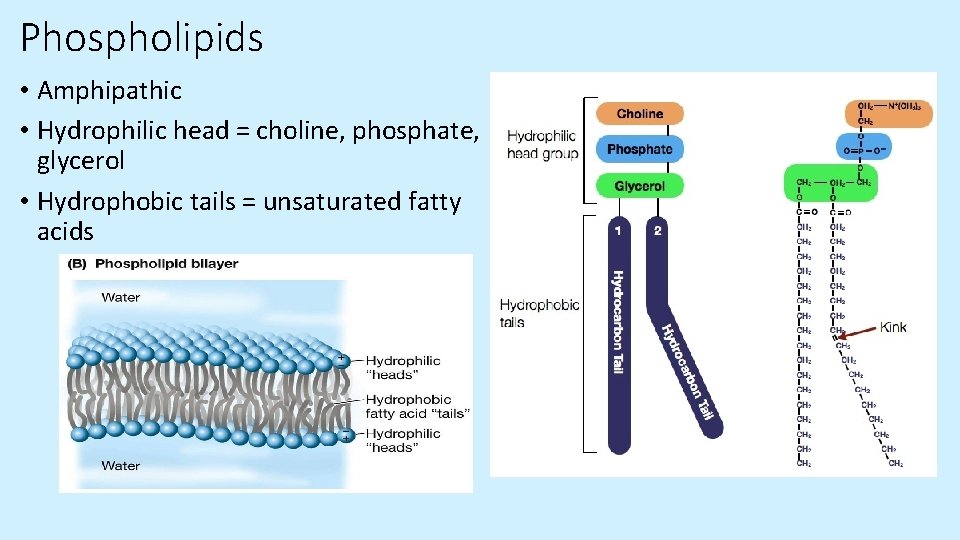
Phospholipids • Amphipathic • Hydrophilic head = choline, phosphate, glycerol • Hydrophobic tails = unsaturated fatty acids
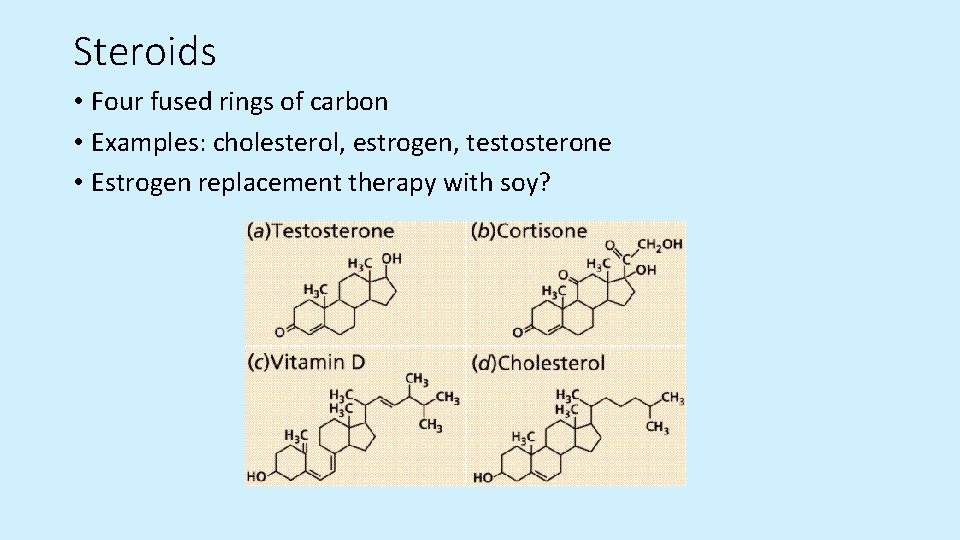
Steroids • Four fused rings of carbon • Examples: cholesterol, estrogen, testosterone • Estrogen replacement therapy with soy?

Proteins • Elements: C, H, O, N, and sometimes S • Many possible functions (not included in the notes) -transport (hemoglobin in our red blood cells) -structure (keratin in our hair and nails) -defense (antibodies in our immune system) -movement (muscle proteins) -signaling (protein hormones) -speeding up reactions (enzymes)
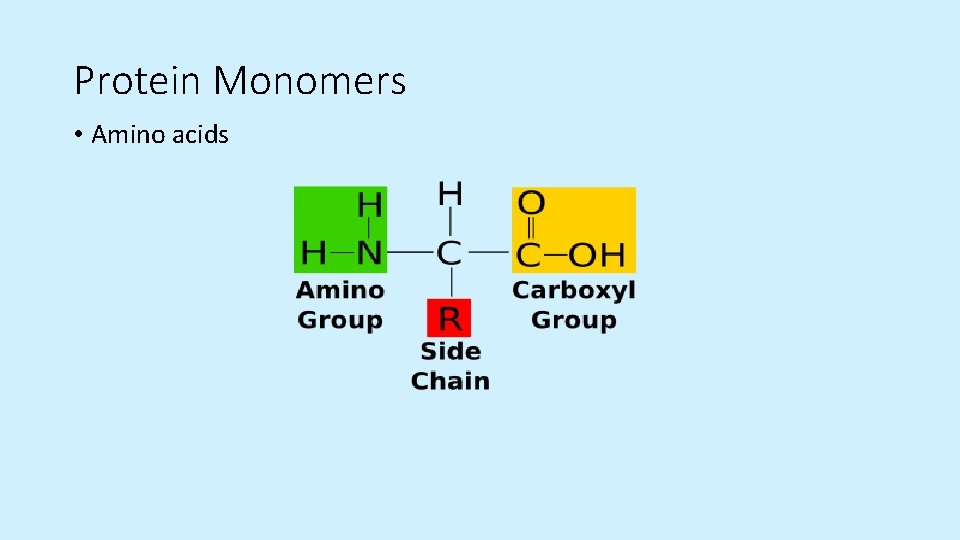
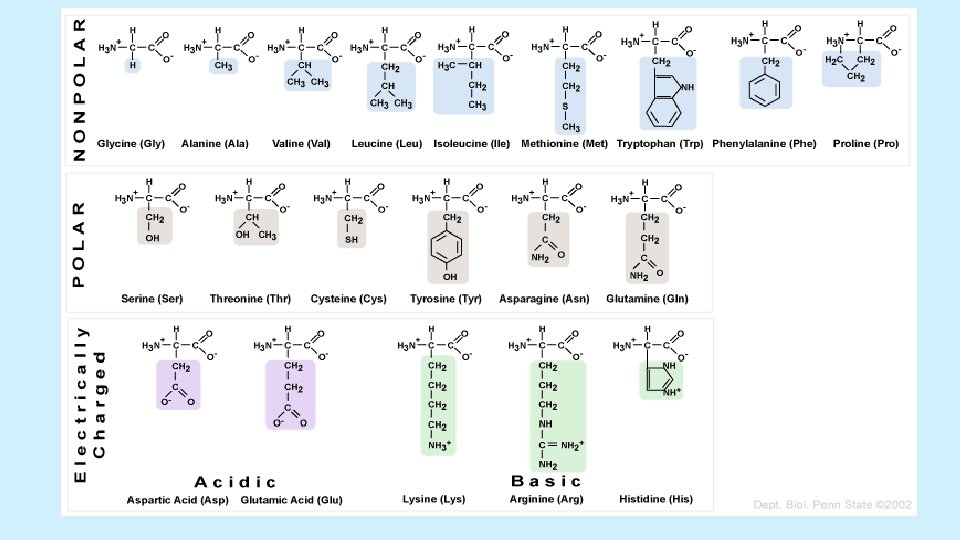
Protein Monomers • Amino acids
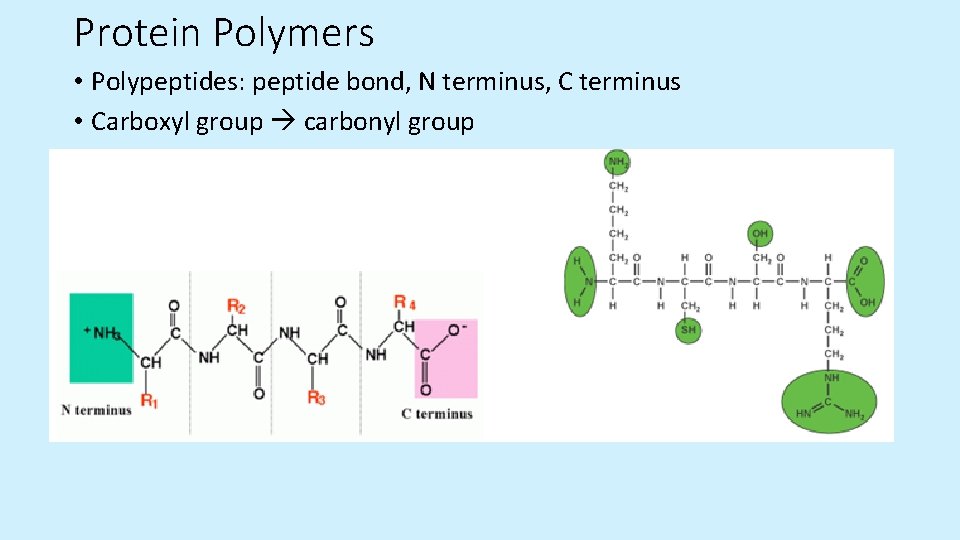
Protein Polymers • Polypeptides: peptide bond, N terminus, C terminus • Carboxyl group carbonyl group
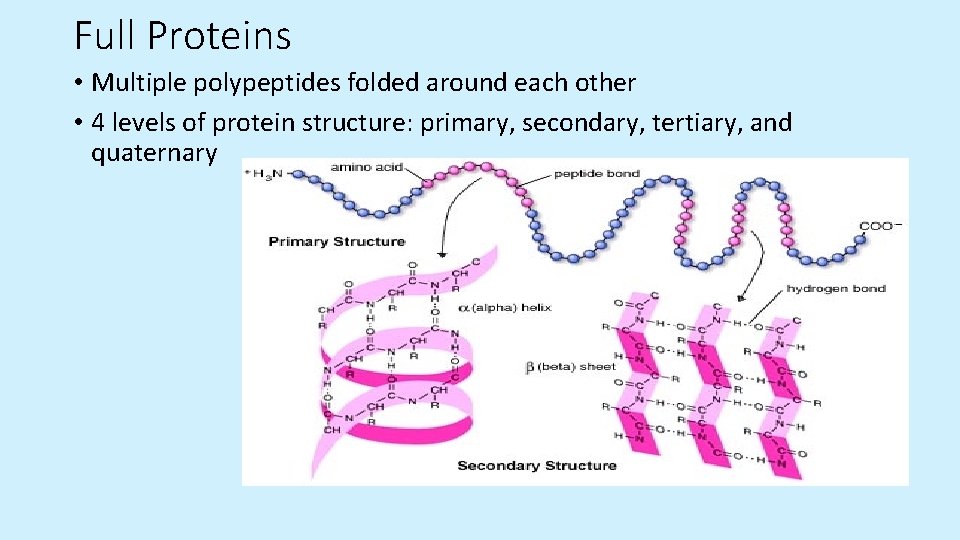
Full Proteins • Multiple polypeptides folded around each other • 4 levels of protein structure: primary, secondary, tertiary, and quaternary
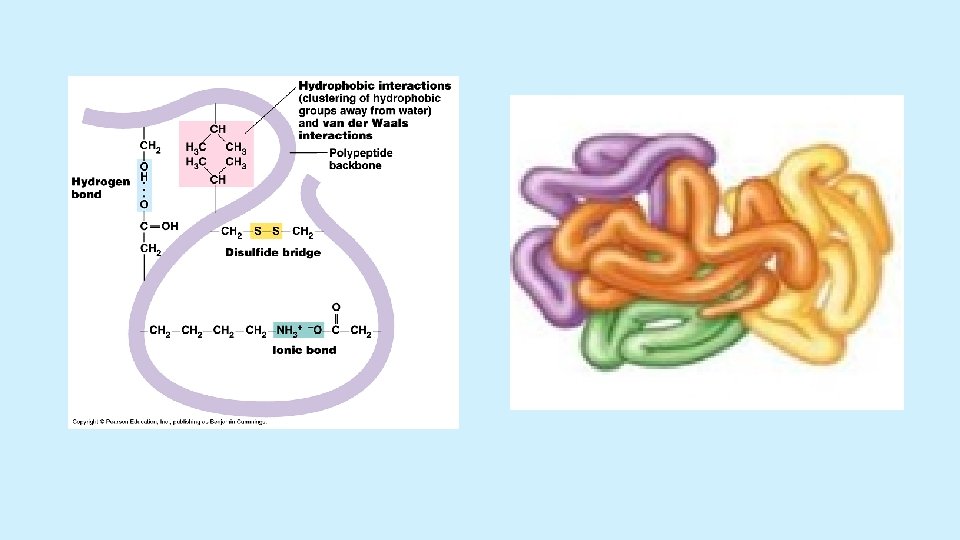
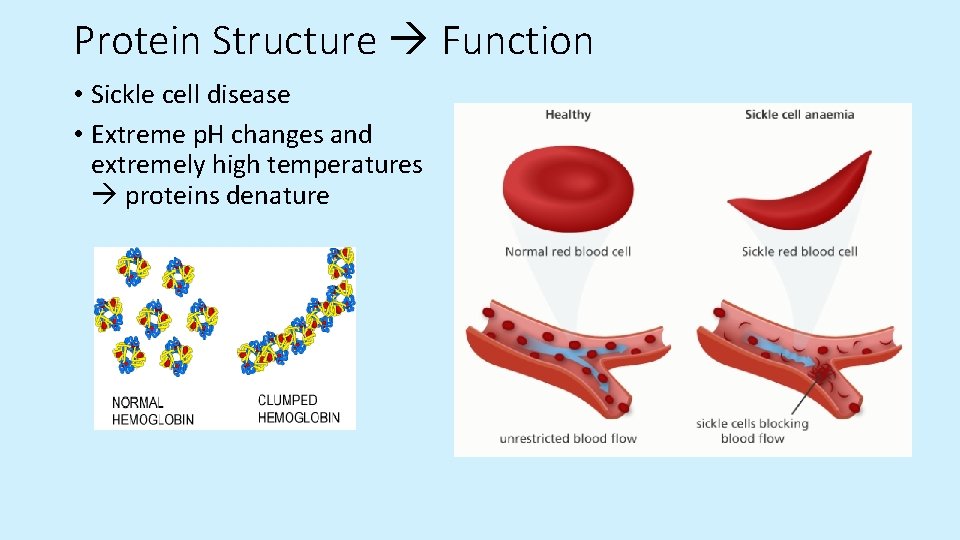
Protein Structure Function • Sickle cell disease • Extreme p. H changes and extremely high temperatures proteins denature
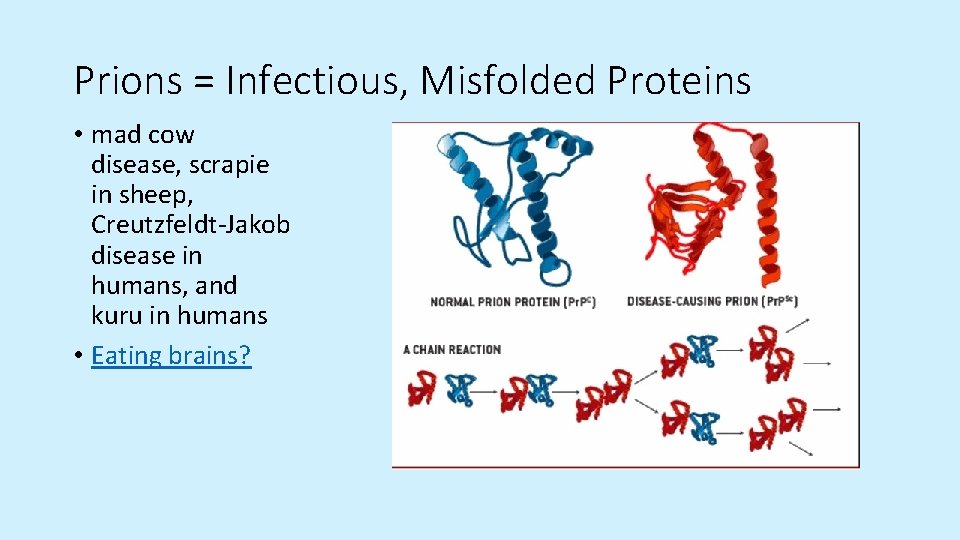
Prions = Infectious, Misfolded Proteins • mad cow disease, scrapie in sheep, Creutzfeldt-Jakob disease in humans, and kuru in humans • Eating brains?

Nucleic Acids • Elements: C, H, O, N, and P • Functions (not in the notes): storing and sending genetic information
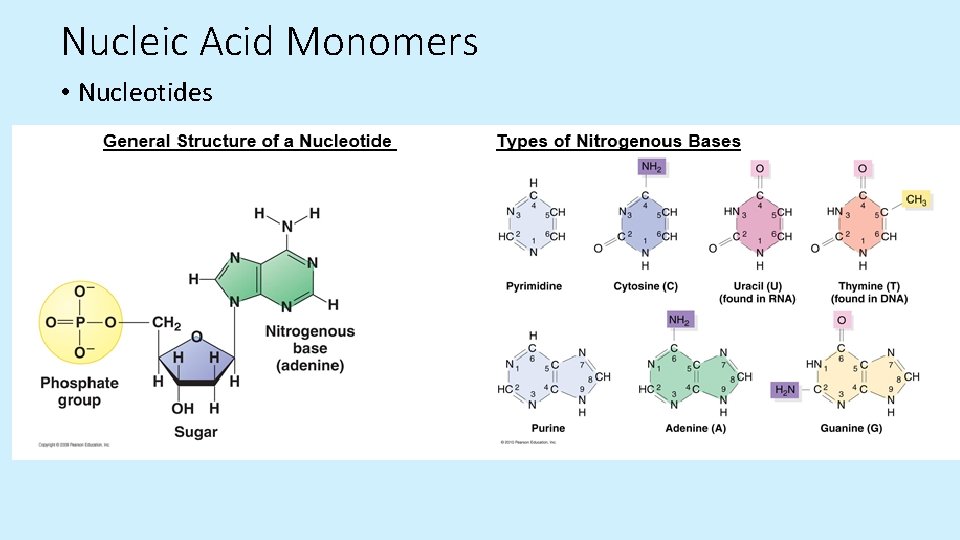
Nucleic Acid Monomers • Nucleotides
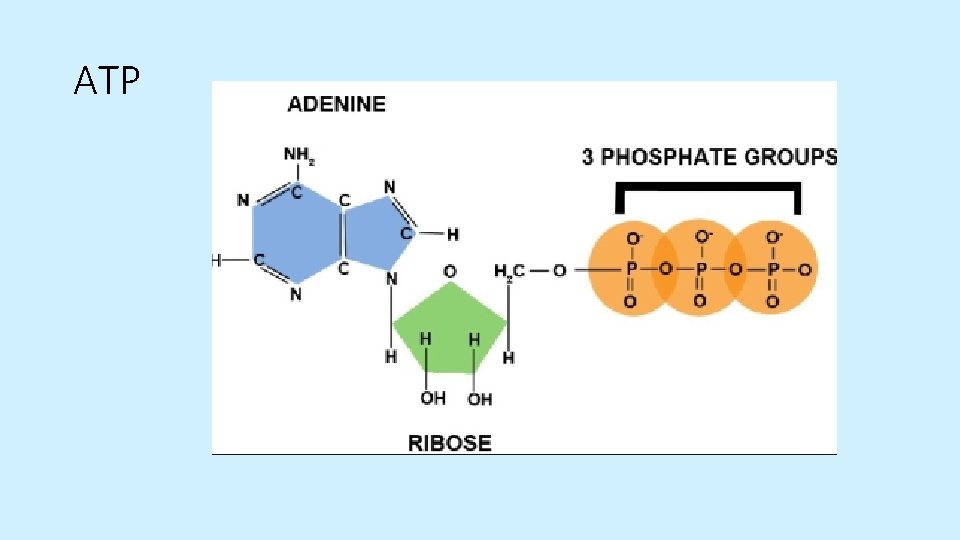
ATP
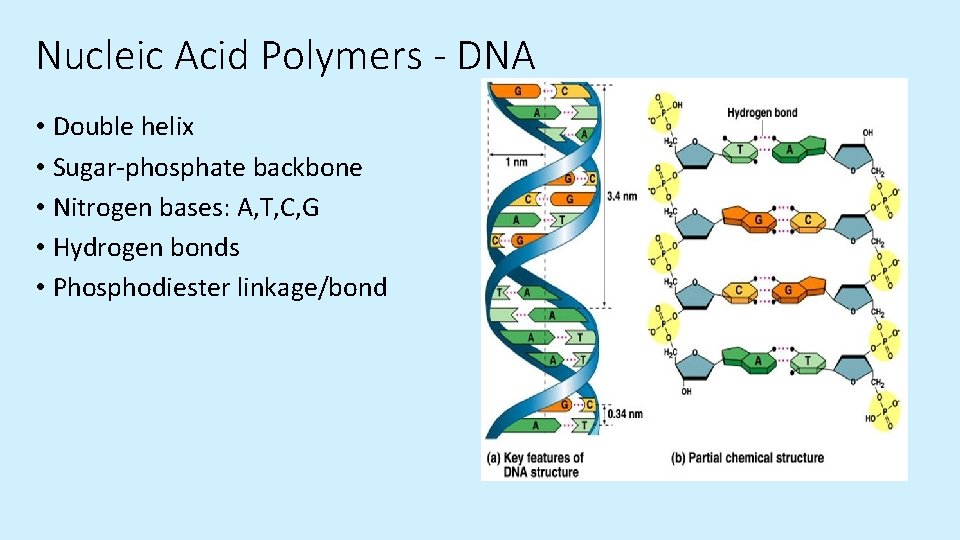
Nucleic Acid Polymers - DNA • Double helix • Sugar-phosphate backbone • Nitrogen bases: A, T, C, G • Hydrogen bonds • Phosphodiester linkage/bond
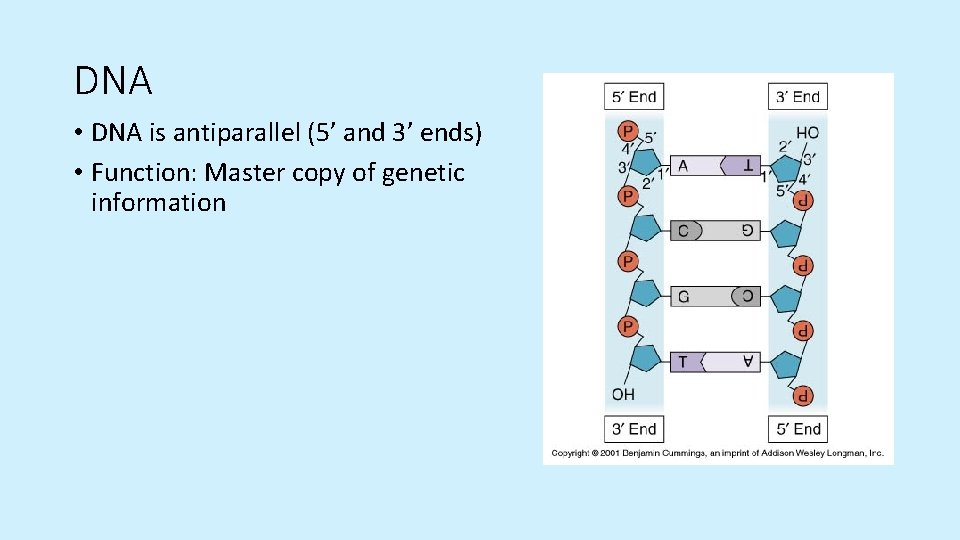
DNA • DNA is antiparallel (5’ and 3’ ends) • Function: Master copy of genetic information
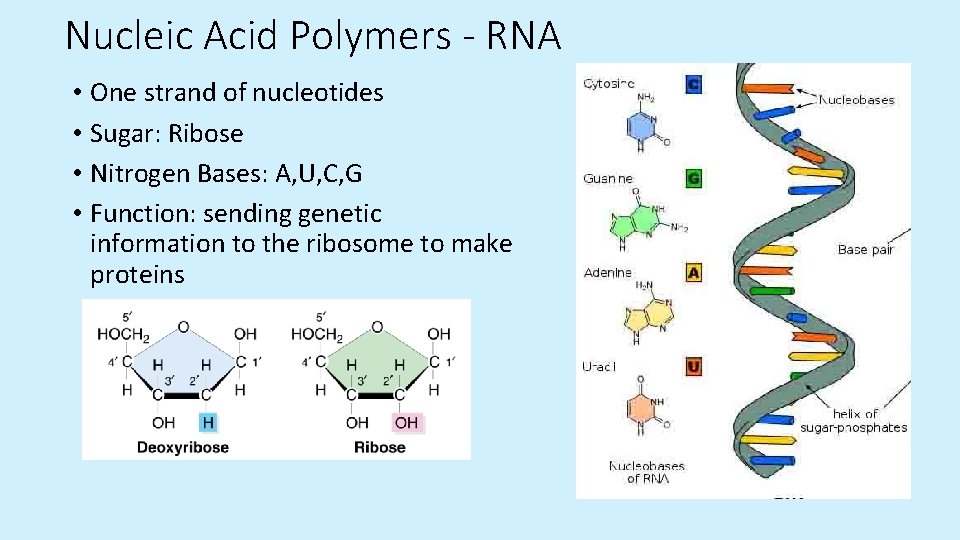
Nucleic Acid Polymers - RNA • One strand of nucleotides • Sugar: Ribose • Nitrogen Bases: A, U, C, G • Function: sending genetic information to the ribosome to make proteins
Macromolecule Flashcards • On the front: 1. Write the name of the Macromolecule Category (Carbohydrate, Lipid, etc. ) 2. Draw a monomer and a polymer • On the back: 1. 2. 3. 4. 5. Write the elements present in this macromolecule Write the macromolecule’s function Write its monomer and example(s) – you can draw it again if you think it’ll help Write its polymer and example(s) – you can draw it again if you think it’ll help Write the name of the bond found between monomers when they join together to make polymers.
- Slides: 43