Unit 2 Nuclear Processes Introduction to Nuclear Radiation























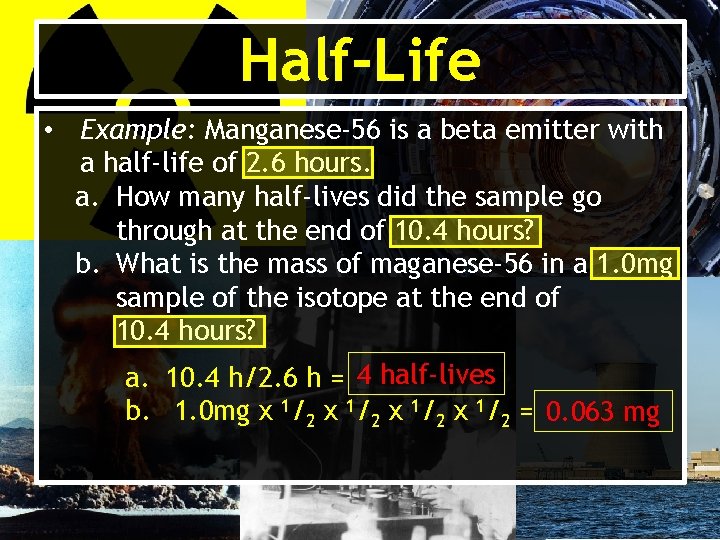


![al∙che∙my [al-kuh-mee] noun: a science that was used in the Middle Ages with the al∙che∙my [al-kuh-mee] noun: a science that was used in the Middle Ages with the](https://slidetodoc.com/presentation_image_h2/befe2a772b87595c90a8a6b9fbd94344/image-37.jpg)





















- Slides: 70

Unit 2: Nuclear Processes Introduction to Nuclear Radiation

Fukishima Nuclear Plant Explosion • BBC Report • CNN Videos http: //www. opensourceinvestigations. com/wp-content/uploads/2015/12/fukusima-accident. jpg

After today you will be able to… • Explain how an unstable nucleus releases energy. • Differentiate between chemical and nuclear reactions. • Identify the three types of nuclear radiation and their properties.

A quick review… Isotope: Atoms of the same element (same atomic number) with different numbers of neutrons (different mass numbers). Examples: Carbon-12, Carbon-13

A quick review… Atomic number: The number of protons in a nucleus. • This cannot change during chemical reactions. Mass number: The number of protons + neutrons in the nucleus of an atom.

A quick review… A shorthand way of indicating the mass number and atomic number for an atom: Mass number Atomic number 12 6 C

Radioactivity • Was first observed by Marie and Pierre Curie (1900 s) using uranium (U) atoms. • Radioactivity: The process by which substances spontaneously emit rays and particles (radiation).

Radioactivity differs from chemical reactions in a number of ways: 1. In chemical reactions, atoms attain a stable electron configuration by losing/sharing electrons. In nuclear reactions, the nucleus of an unstable isotope gains stability by undergoing changes.

Radioactivity differs from chemical reactions in a number of ways: 2. Unlike chemical reactions, nuclear reactions are not affected by changes in temperature, pressure, or the presence of catalysts. 3. Nuclear reactions of an isotope cannot be sped up, slowed down, or turned off.

Radioactivity • Unstable radioactive isotopes (radioisotopes) are transformed into stable (nonradioactive) isotopes of a different element. • Radioactive decay: is the process by which an unstable nucleus emits radiation and energy in order to obtain a more stable state.

Types of Radiation: 1. Alpha radiation 2. Beta radiation 3. Gamma radiation

Alpha (α) Radiation • Recall, Consists Rutherford of helium nuclei that has been emitted from a radioactive used alpha particles source. to discover the • Contains two protons, two neutrons, nucleus his Gold and has ain positive two charge. • Foil Usually written as: He or α (the Experiment. 4 electric charge is usually omitted). 2

Alpha (α) Radiation • Due to its large mass and charge, alpha particles do not travel very far. • They cannot penetrate very well – a sheet of paper or the surface of your skin stops them. • However, they are toxic when ingested.

Alpha (α) Radiation Example: Alpha decay of Uranium-238 92 U Radioactive decay 234 90 4 2 Th + He

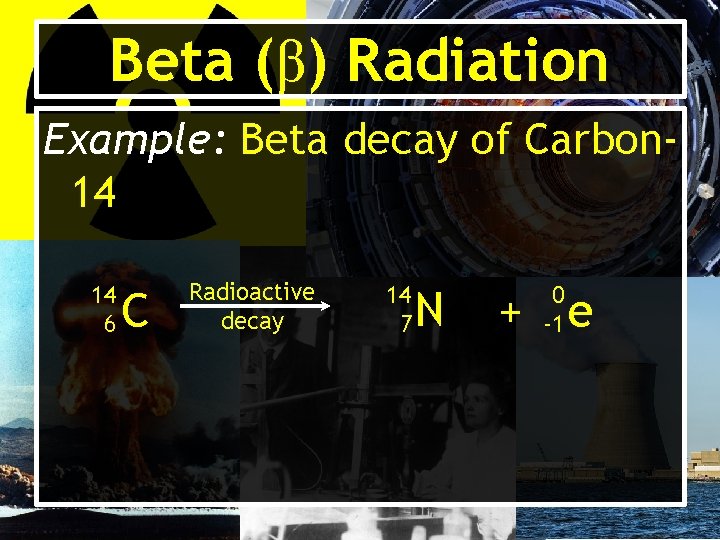
Beta (β) Radiation • Results from the breaking apart of a neutron into a proton and an electron (beta particle). • Usually written as: -10 e or β • Has much less mass than an alpha particle and can therefore penetrate more easily. • Can pass through paper, but is stopped by foil or wood.

Beta (β) Radiation Example: Beta decay of Carbon 14 14 6 C Radioactive decay 14 7 N 0 -1 + e

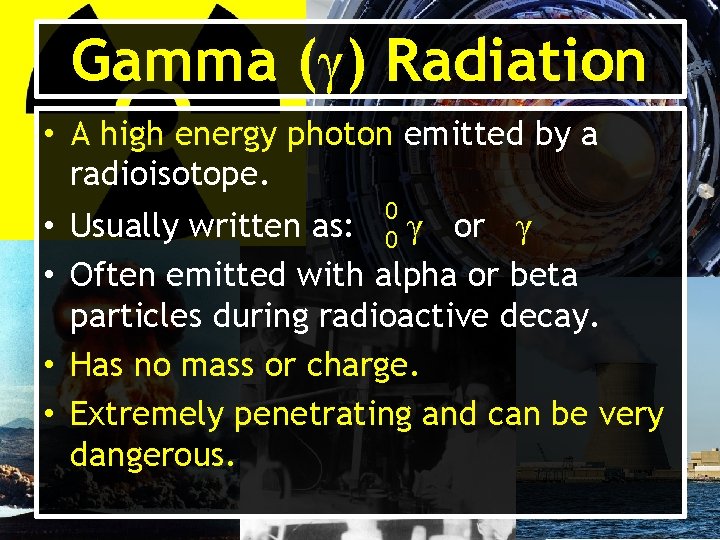
Gamma (γ) Radiation • A high energy photon emitted by a radioisotope. 0 • Usually written as: 0 γ or γ • Often emitted with alpha or beta particles during radioactive decay. • Has no mass or charge. • Extremely penetrating and can be very dangerous.

Gamma (γ) Radiation • Easily passes through paper, wood, and the human body. • Penetration can be stopped, although not completely, by several centimeters of lead.

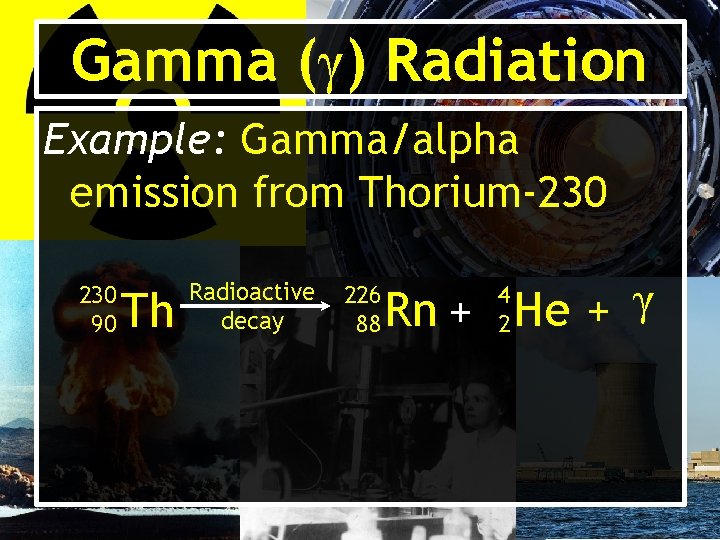
Gamma (γ) Radiation Example: Gamma/alpha emission from Thorium-230 90 Th Radioactive decay 226 88 Rn + He + γ 4 2

Questions? Complete the “Learned” portion of your table from the bellwork today. Then hand it in!

Unit 2: Nuclear Processes Half-Life

After today you will be able to… • Identify the factor that nuclear stability is dependent on. • Calculate the half-life for a given radioisotope. • Calculate how much of a radioisotope remains after a given amount of time.

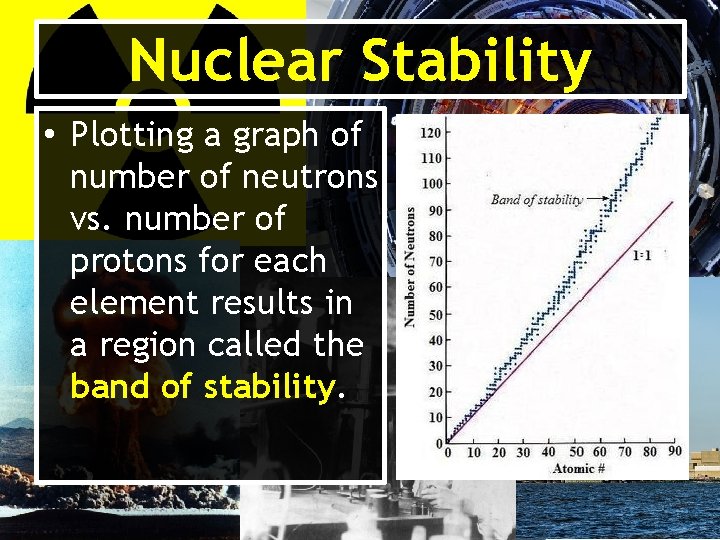
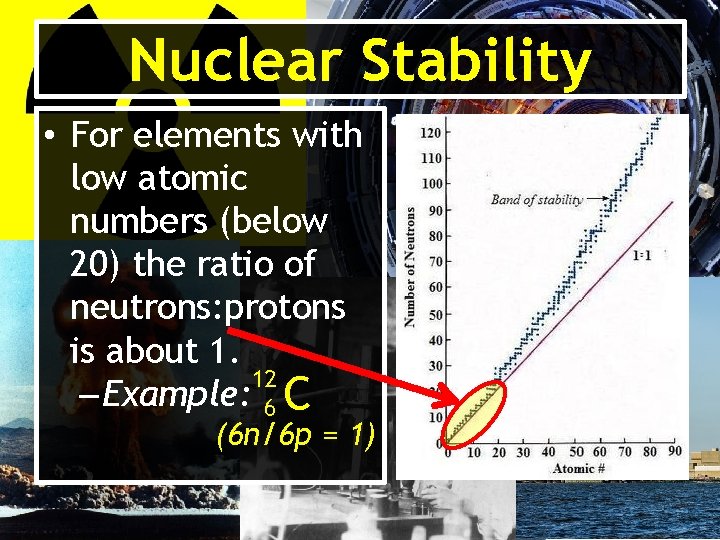
Nuclear Stability • Close to 2, 000 different nuclei are known. • Approximately 260 are stable and do not decay or change with time. • The stability (resistance to change) depends on its neutron: proton ratio.

Nuclear Stability • Plotting a graph of number of neutrons vs. number of protons for each element results in a region called the band of stability.

Nuclear Stability • For elements with low atomic numbers (below 20) the ratio of neutrons: protons is about 1. 12 – Example: 6 C (6 n/6 p = 1)

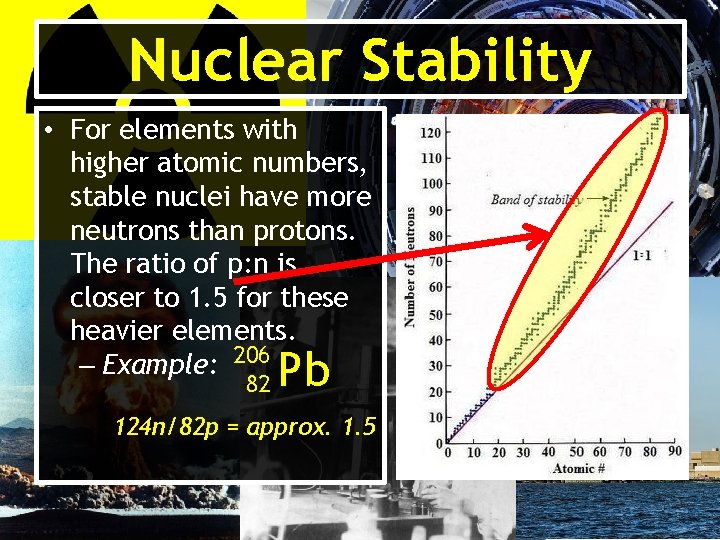
Nuclear Stability • For elements with higher atomic numbers, stable nuclei have more neutrons than protons. The ratio of p: n is closer to 1. 5 for these heavier elements. – Example: 206 82 Pb 124 n/82 p = approx. 1. 5

Nuclear Stability • The neutron: proton ratio iswhy Ever wonder important because it some atomic masses determineslisted the type of Periodic decay on the that occurs. Table have ( ) around • All nuclei that have an atomic them? Because their number greater thanmasses 83 areare atomic radioactive. estimated due to radioactive decay!

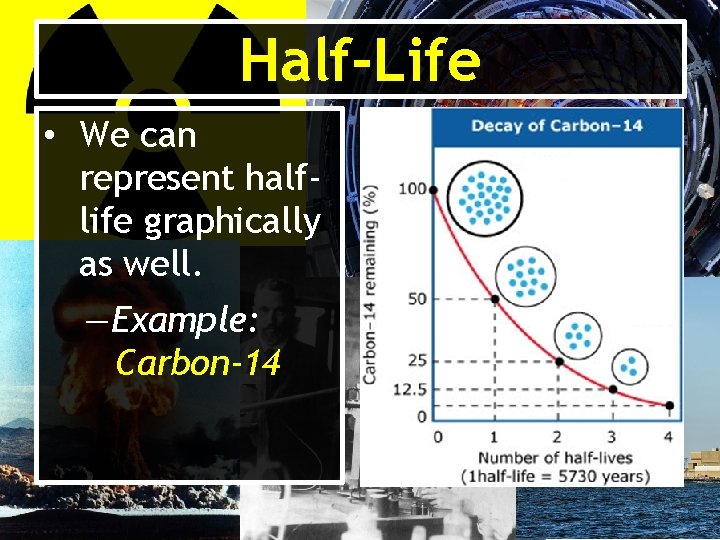
Half-Life Half-life: (t 1/2) the time required for half of the nuclei of a radioisotope sample to decay to products. • Example: If you have 20 atoms of Radon 222, the half life is ~4 days. How many atoms remain at the end of two half lives? 0 t 1/2 20 atoms initially 4 days 1 t 1/2 10 atoms 8 days 2 t 1/2 5 atoms remain

Half-Life • We can represent halflife graphically as well. —Example: Carbon-14

However, very seldom do we count atoms. Therefore it is more appropriate to calculate amount that remains in terms of mass.

Half-Life • Example: Carbon-14 emits beta radiation and decays with a half-life (t 1/2) of 5730 years. Assume you start with a mass of 2. 00 x 10 -12 g of carbon-14. a. How long is three half-lives? b. How many grams of the isotope remain at the end of three half-lives? a. 3(5730) = 17, 190 years b. 2. 00 x 10 -12 g x 1/2 = 2. 5 x 10 -13 g

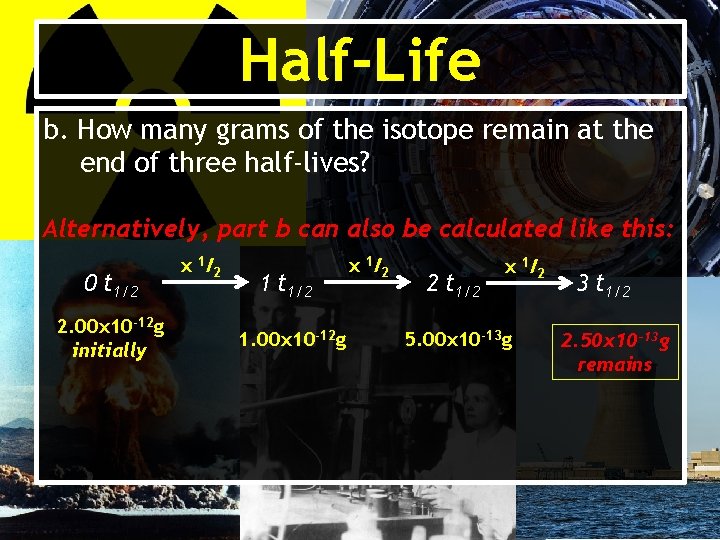
Half-Life b. How many grams of the isotope remain at the end of three half-lives? Alternatively, part b can also be calculated like this: 0 t 1/2 2. 00 x 10 -12 g initially x 1 /2 1 t 1/2 1. 00 x 10 -12 g x 1 /2 2 t 1/2 x 1 /2 5. 00 x 10 -13 g 3 t 1/2 2. 50 x 10 -13 g remains
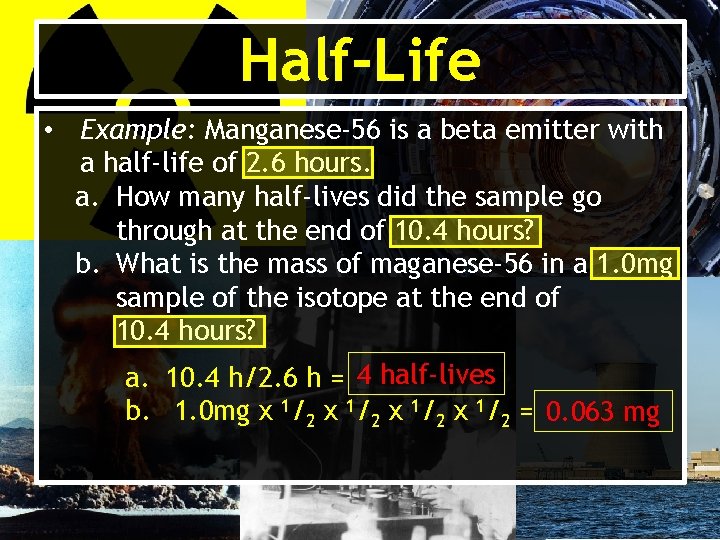
Half-Life • Example: Manganese-56 is a beta emitter with a half-life of 2. 6 hours. a. How many half-lives did the sample go through at the end of 10. 4 hours? b. What is the mass of maganese-56 in a 1. 0 mg sample of the isotope at the end of 10. 4 hours? a. 10. 4 h/2. 6 h = 4 half-lives b. 1. 0 mg x 1/2 = 0. 063 mg

Questions? Complete and turn in the exit ticket.

Unit 2: Nuclear Processes Transmutations

After today you will be able to… • Identify and explain two ways in which transmutations can occur. • Balance nuclear reactions.
![alchemy alkuhmee noun a science that was used in the Middle Ages with the al∙che∙my [al-kuh-mee] noun: a science that was used in the Middle Ages with the](https://slidetodoc.com/presentation_image_h2/befe2a772b87595c90a8a6b9fbd94344/image-37.jpg)
al∙che∙my [al-kuh-mee] noun: a science that was used in the Middle Ages with the goal of changing ordinary metals into gold -Courtesy of Merriam-Webster (2015)

A brief history… • The modern practice of chemistry started in medieval Europe and the Middle East. • Alchemists (~16 th century) believed that by doing certain chemical reactions, you could turn cheap metals into gold. • We now know that no chemical reaction can achieve this goal.

However, through transmutations modern chemists can change one element into another.

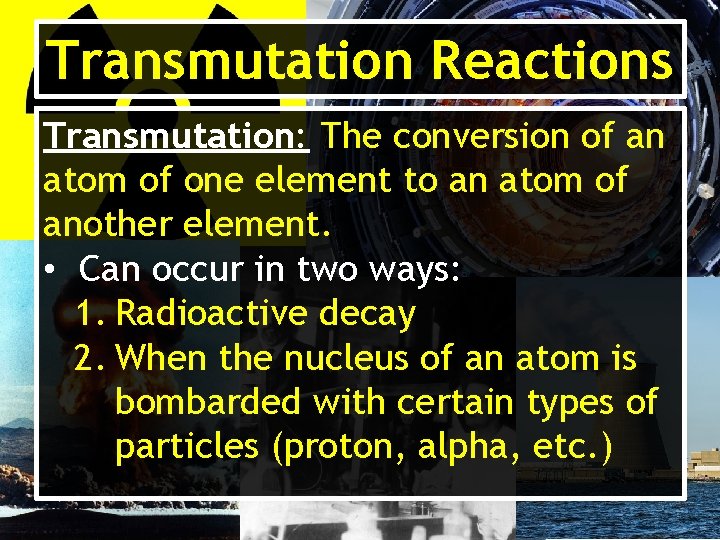
Transmutation Reactions Transmutation: The conversion of an atom of one element to an atom of another element. • Can occur in two ways: 1. Radioactive decay 2. When the nucleus of an atom is bombarded with certain types of particles (proton, alpha, etc. )

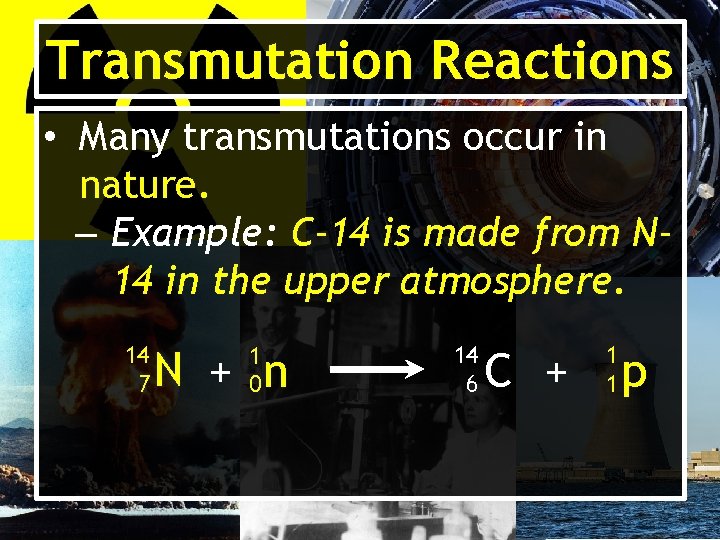
Transmutation Reactions • Many transmutations occur in nature. – Example: C-14 is made from N 14 in the upper atmosphere. 14 7 1 0 N + n 14 6 C + 1 1 p

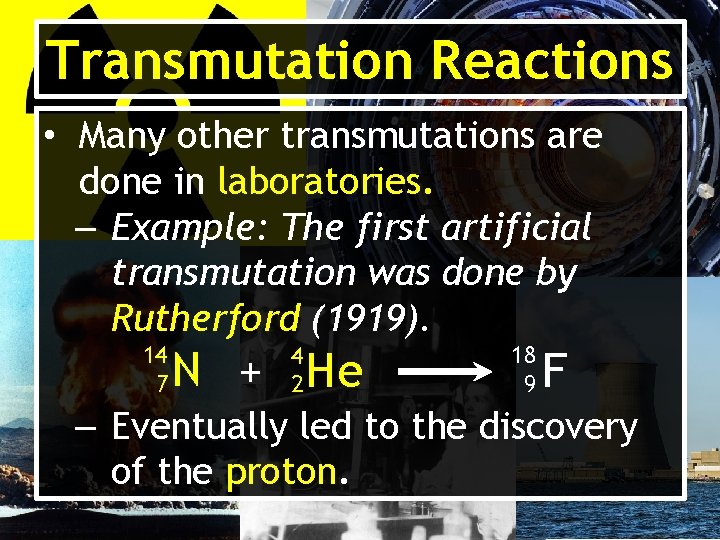
Transmutation Reactions • Many other transmutations are done in laboratories. – Example: The first artificial transmutation was done by Rutherford (1919). 14 7 4 2 N + He 18 9 F – Eventually led to the discovery of the proton.

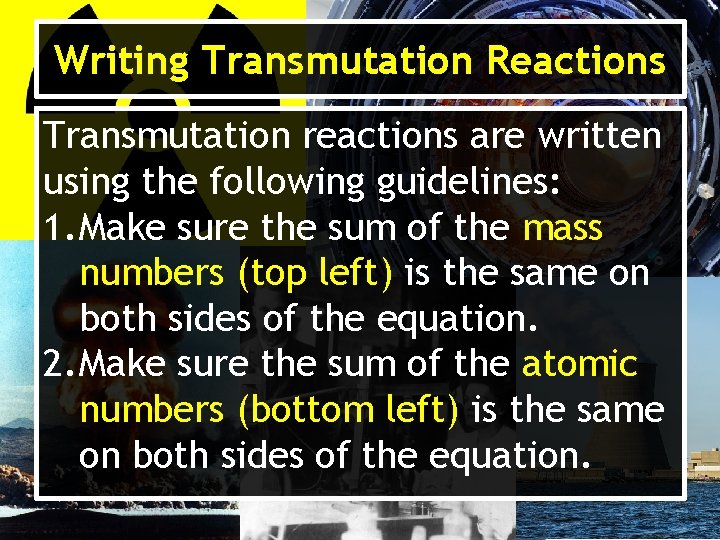
Writing Transmutation Reactions Transmutation reactions are written using the following guidelines: 1. Make sure the sum of the mass numbers (top left) is the same on both sides of the equation. 2. Make sure the sum of the atomic numbers (bottom left) is the same on both sides of the equation.

3. Be familiar with the following particles and their notations: Particle Alpha Beta Gamma Neutron Proton Positron Symbol 4 2 He or 0 or -1 e 0 0γ 1 0 n 1 or 1 H 0 or +1 e 4 2 α 0 -1 β 1 1 p 0 +1 β

Writing Transmutation Reactions Example: Identify the missing information. 239 94 4 2 Pu + He ? 242 96 1 0 Cm ? + n

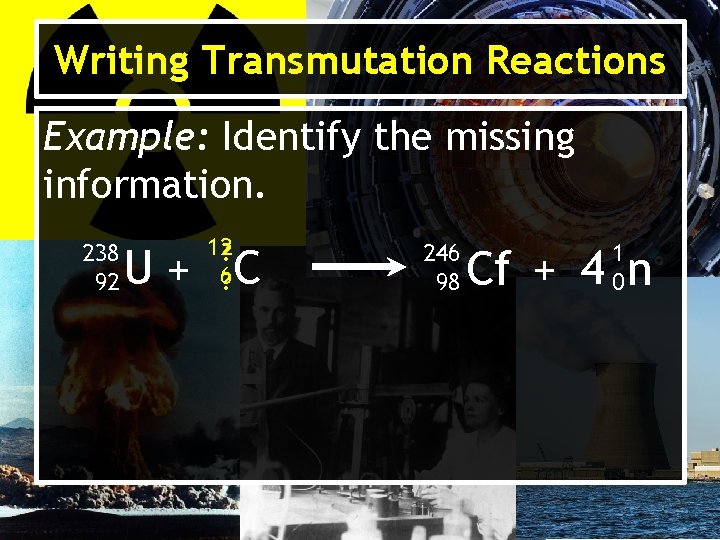
Writing Transmutation Reactions Example: Identify the missing information. 238 92 12? 6? U+ C 246 98 1 0 Cf + 4 n

Writing Transmutation Reactions Example: Write the beta decay for carbon-14. 14 6 C 0 -1 e + 14 7 N

Writing Transmutation Reactions Example: Write the alpha decay for radon-222. 222 86 Rn 4 2 He + 218 84 Po

Transuranium Elements • Elements in the Periodic Table with atomic numbers above 92 are called transuranium elements. • All of these elements undergo transmutation, do not occur in nature, and are synthesized in the laboratory. • To synthesize these elements, particle accelerators bombard nuclei with fast moving particles.

Particle Accelerator:

Questions? Begin WS 3

Unit 2: Nuclear Processes Fission, Fusion, and Applications of Nuclear Chemistry

After today you will be able to… • Describe what happens in a nuclear chain reaction. • Differentiate between fission and fusion reactions. • Identify examples of how radioisotopes are used in everyday life.

Recall, during transmutations an atom can be converted into a different atom through radioactive decay or by bombarding a nucleus with particles. Today, we will focus on the latter by learning about fission and fusion.

Nuclear Fission: occurs when a nucleus is split into smaller pieces. • This process begins by colliding a nucleus with particles such as neutrons. • The resulting nucleus is highly unstable and decays. • This causes a chain reaction where particles emitted from the initial reaction bombard other nuclei, causing them to break apart.

Example: Uranium-235 1 0 n 235 The neutrons released U 92 from the decay of U-236 d. e orm will collide with other f s i 36 Kr 2 U n 236 U-235 atoms, repeating 92 U ENERGY n The U-236 atom is this process all over unstable and deca ys. n again (chain reaction). Ba A neutron collid es with the U-235 atom. 91 36 1 0 142 56 1 0

Nuclear Fission • Uranium-235 and Plutonium -239 are the only fissionable isotopes. • These reactions release a large amount of energy.

Nuclear Fusion: occurs when nuclei combine (or fuse together) to produce a new nucleus of greater mass. • Example: Production of energy from the sun 1 1 4 H 4 2 He + 2 e + 0 +1 energy

Nuclear Fusion • Releases far more energy than fission reactions. • These reactions require very high temperatures in order to occur (~40, 000°C).

Real-World Applications: C-14 Dating Scientists often find the age of an object that was once living by measuring the amount of carbon-14 it contains. • Recall, C-14 has a half-life of 5730 years. • This is its nuclear decay: 14 6 C 14 7 N + 0 -1 e

Real-World Applications: C-14 Dating • All living things contain C-12 and C-14 in a fixed ratio. • Once an organism dies, the ratio of C-14: C-12 changes, which allows archeologists to estimate its age.

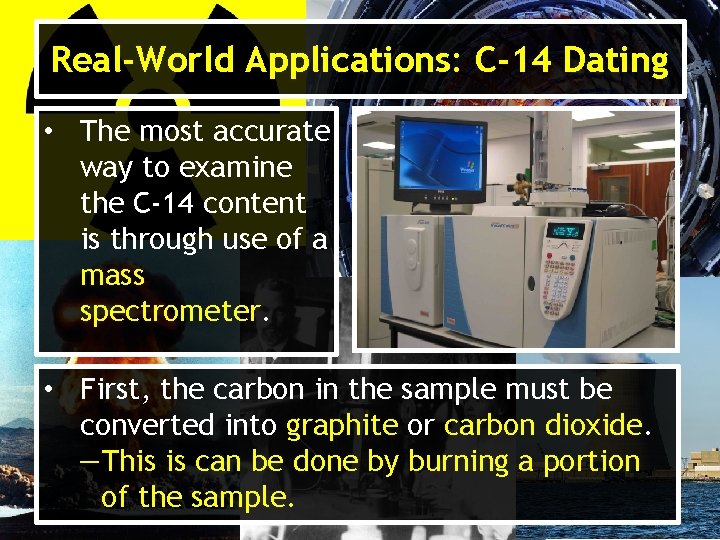
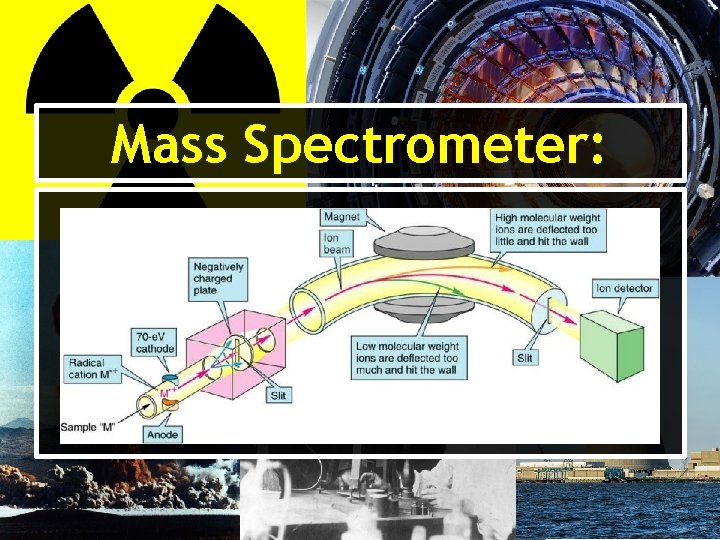
Real-World Applications: C-14 Dating • The most accurate way to examine the C-14 content is through use of a mass spectrometer. • First, the carbon in the sample must be converted into graphite or carbon dioxide. ―This is can be done by burning a portion of the sample.

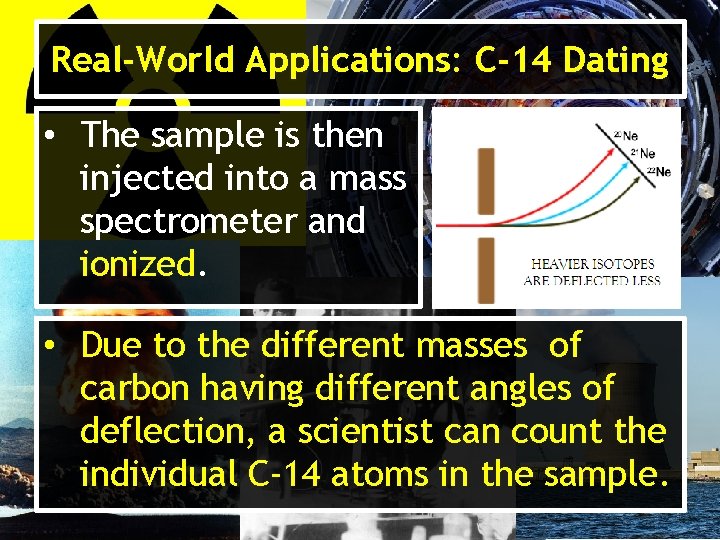
Real-World Applications: C-14 Dating • The sample is then injected into a mass spectrometer and ionized. • Due to the different masses of carbon having different angles of deflection, a scientist can count the individual C-14 atoms in the sample.

Mass Spectrometer:

Real-World Applications: U-238 But what if all of the C-14 in a sample has decayed or the sample is non-living? • Similar to carbon-14, uranium-238 is used to date specimens. • Uranium is naturally found in most rocks, seawater, and in Earth’s crust. • It has a half-life of 4. 5 billion years. • U-238 is specifically used in dating rocks and fossils.

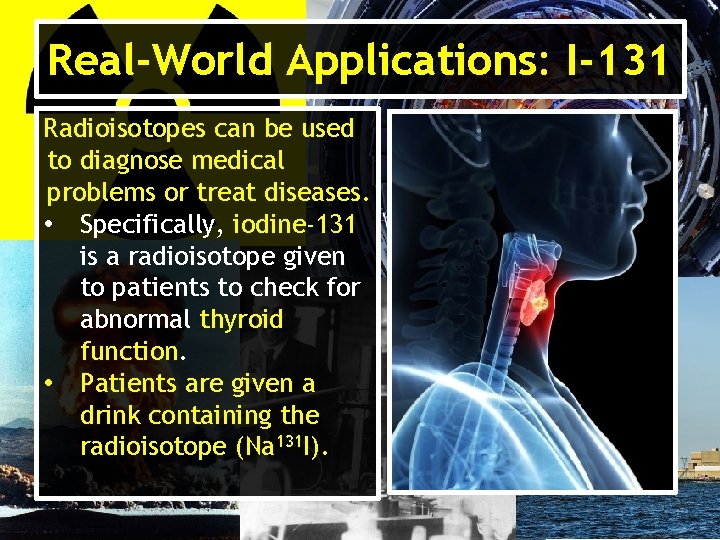
Real-World Applications: I-131 Radioisotopes can be used to diagnose medical problems or treat diseases. • Specifically, iodine-131 is a radioisotope given to patients to check for abnormal thyroid function. • Patients are given a drink containing the radioisotope (Na 131 I).

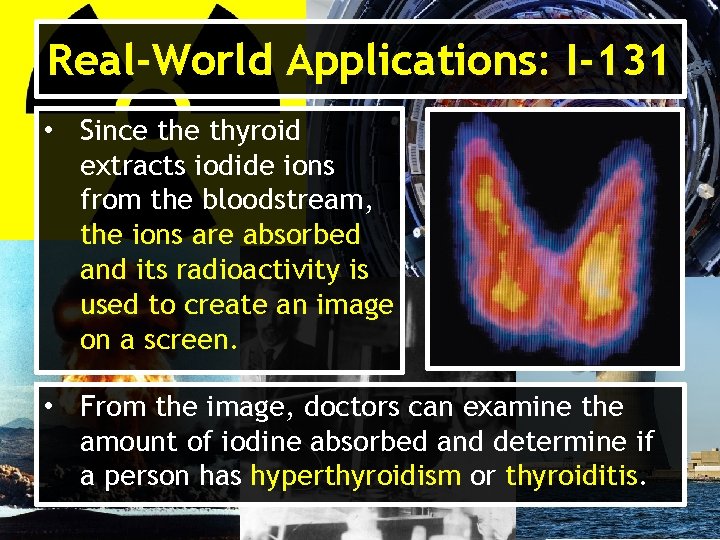
Real-World Applications: I-131 • Since thyroid extracts iodide ions from the bloodstream, the ions are absorbed and its radioactivity is used to create an image on a screen. • From the image, doctors can examine the amount of iodine absorbed and determine if a person has hyperthyroidism or thyroiditis.

Real-World Applications: Co-60 The strong penetrating power of gamma rays allows it to be useful in the treatment of cancer. • Cobalt-60, which is produced in particle accelerators, emits both beta and gamma radiation.

Real-World Applications: Co-60 • The Co-60 is placed into a gun that is used to direct the radiation to where the tumor is located. • The cells of the tumor are destroyed and it decreases in size. • However, gamma radiation can also destroy healthy cells making those treated very ill.

Questions? Begin WS 4