UNIT 2 MECHANICS CHAPTER 8 FLUID MECHANICS Chapter












- Slides: 23

UNIT 2 - MECHANICS CHAPTER 8 - FLUID MECHANICS

Chapter 8 C Gas Laws

Lots of Scientists in this Chapter!! – Guillaume Amontons – John Dalton – Joseph Gay-Lussac – Amedeo Avogadro – Robert Boyle – Jacques Charles

Boyle’s Law • States that the volume of a fixed quantity of a confined gas is inversely proportional to its pressure when its temperature is held constant • Formula: – P 1 V 1=P 2 V 2 – P=pressure V=volume • Example Problem 8 -1 & 8 -2 page 171 • How is this useful? ? – Compressed air?

Charles’s Law • States that the volume of a fixed quantity of a confined gas is directly proportional to its absolute temperature when its pressure is held constant • Formula: – V 1/T 1 = V 2/T 2 – V=volume T=temperature in Kelvin! • How do we get Kelvin from Celsius? ? • Example Problem 8 -3 page 173

UNIT 2 MECHANICS CHAPTER 9 THERMODYNAMICS

CHAPTER 9 B TEMPERATURE

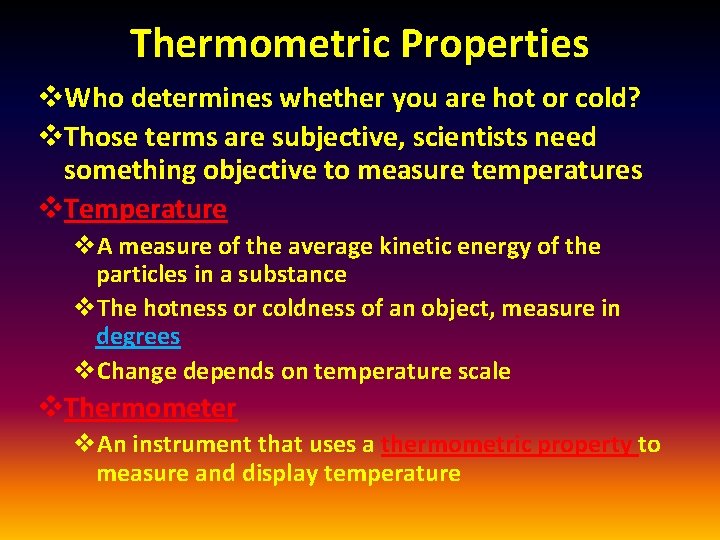
Thermometric Properties v. Who determines whether you are hot or cold? v. Those terms are subjective, scientists need something objective to measure temperatures v. Temperature v. A measure of the average kinetic energy of the particles in a substance v. The hotness or coldness of an object, measure in degrees v. Change depends on temperature scale v. Thermometer v. An instrument that uses a thermometric property to measure and display temperature

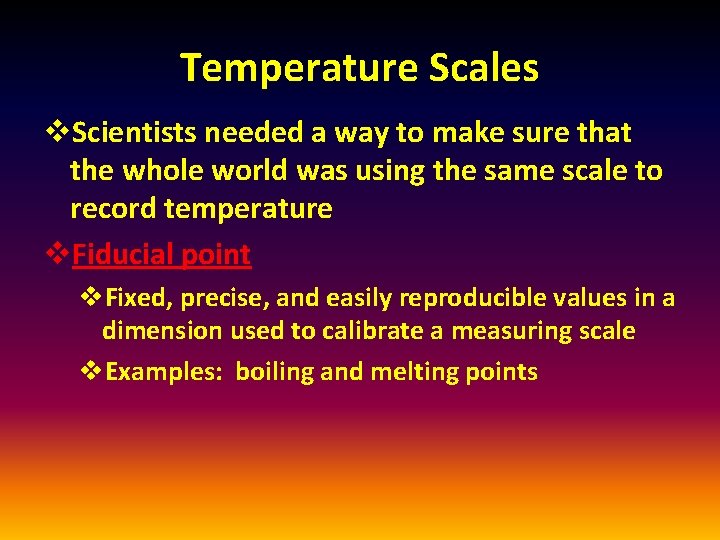
Temperature Scales v. Scientists needed a way to make sure that the whole world was using the same scale to record temperature v. Fiducial point v. Fixed, precise, and easily reproducible values in a dimension used to calibrate a measuring scale v. Examples: boiling and melting points

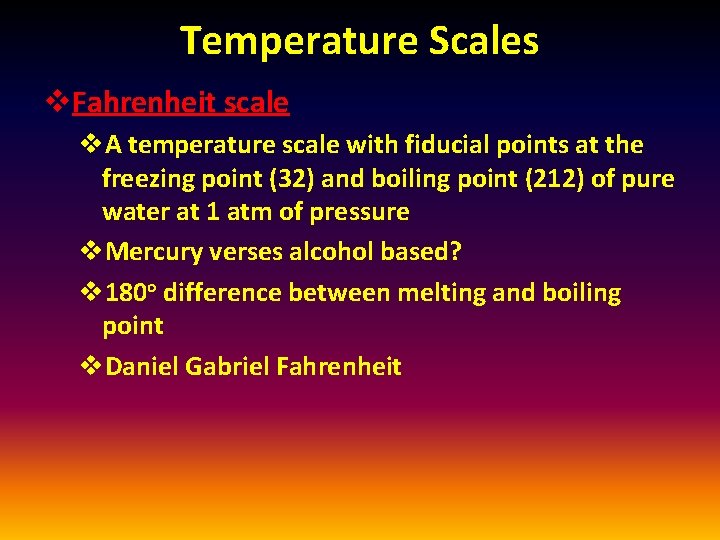
Temperature Scales v. Fahrenheit scale v. A temperature scale with fiducial points at the freezing point (32) and boiling point (212) of pure water at 1 atm of pressure v. Mercury verses alcohol based? v 180 o difference between melting and boiling point v. Daniel Gabriel Fahrenheit

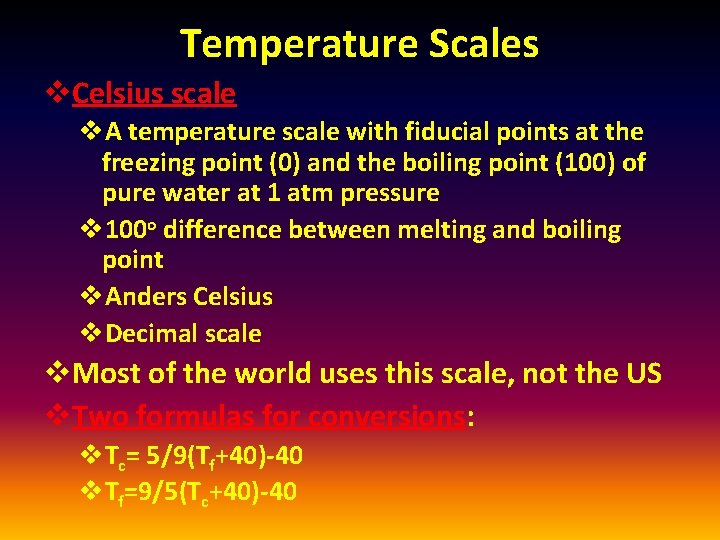
Temperature Scales v. Celsius scale v. A temperature scale with fiducial points at the freezing point (0) and the boiling point (100) of pure water at 1 atm pressure v 100 o difference between melting and boiling point v. Anders Celsius v. Decimal scale v. Most of the world uses this scale, not the US v. Two formulas for conversions: v. Tc= 5/9(Tf+40)-40 v. Tf=9/5(Tc+40)-40

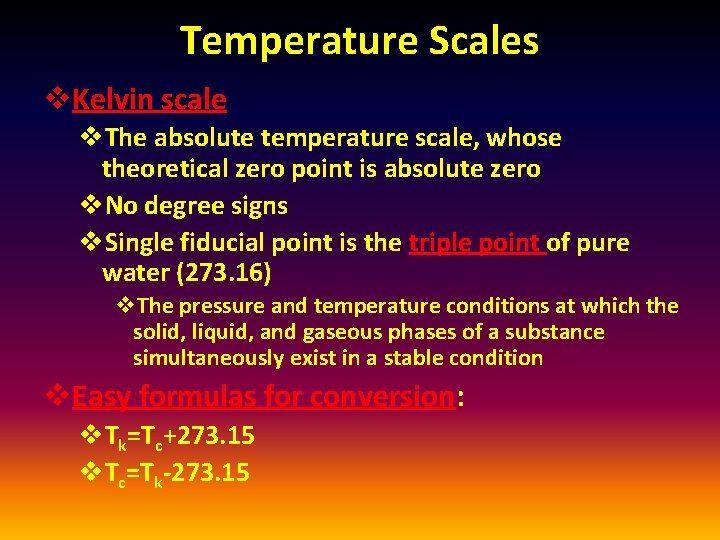
Temperature Scales v. Kelvin scale v. The absolute temperature scale, whose theoretical zero point is absolute zero v. No degree signs v. Single fiducial point is the triple point of pure water (273. 16) v. The pressure and temperature conditions at which the solid, liquid, and gaseous phases of a substance simultaneously exist in a stable condition v. Easy formulas for conversion: v. Tk=Tc+273. 15 v. Tc=Tk-273. 15

CHAPTER 9 C HEAT

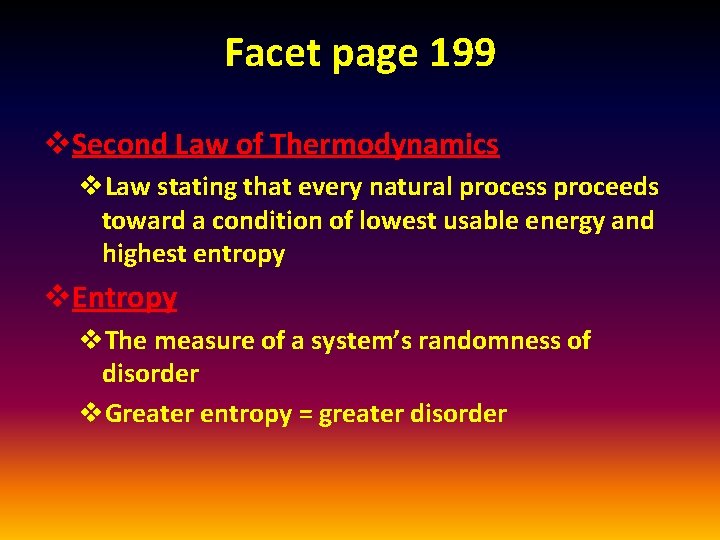
Facet page 199 v. Second Law of Thermodynamics v. Law stating that every natural process proceeds toward a condition of lowest usable energy and highest entropy v. Entropy v. The measure of a system’s randomness of disorder v. Greater entropy = greater disorder

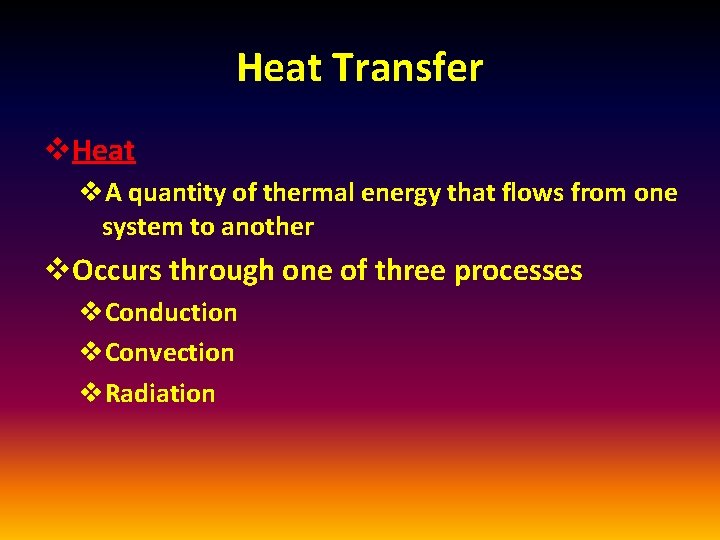
Heat Transfer v. Heat v. A quantity of thermal energy that flows from one system to another v. Occurs through one of three processes v. Conduction v. Convection v. Radiation

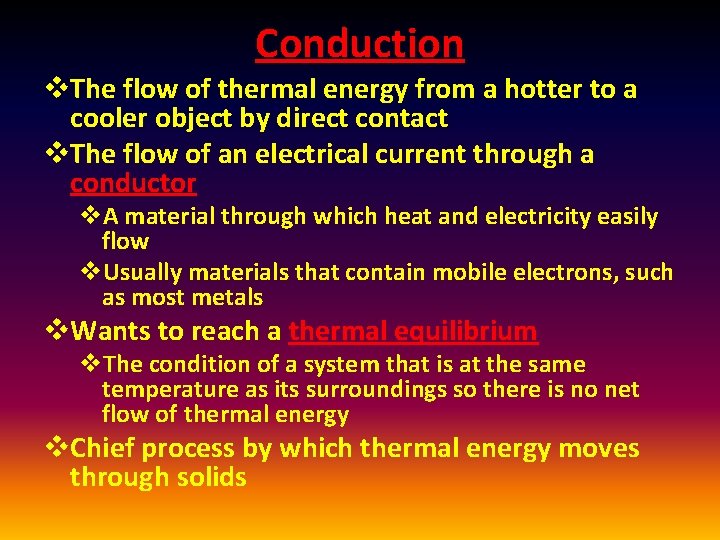
Conduction v. The flow of thermal energy from a hotter to a cooler object by direct contact v. The flow of an electrical current through a conductor v. A material through which heat and electricity easily flow v. Usually materials that contain mobile electrons, such as most metals v. Wants to reach a thermal equilibrium v. The condition of a system that is at the same temperature as its surroundings so there is no net flow of thermal energy v. Chief process by which thermal energy moves through solids

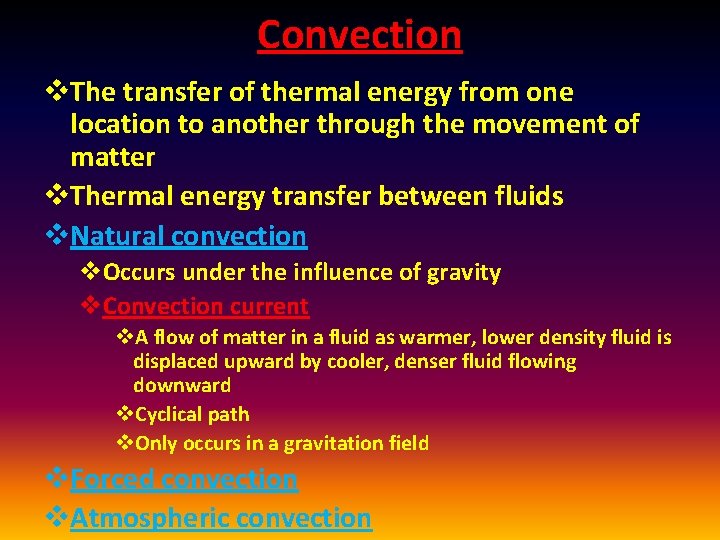
Convection v. The transfer of thermal energy from one location to another through the movement of matter v. Thermal energy transfer between fluids v. Natural convection v. Occurs under the influence of gravity v. Convection current v. A flow of matter in a fluid as warmer, lower density fluid is displaced upward by cooler, denser fluid flowing downward v. Cyclical path v. Only occurs in a gravitation field v. Forced convection v. Atmospheric convection

Radiation v. Nuclear particles or electromagnetic waves that radiate away from their sources v. A method of heat transfer through radiant (electromagnetic) energy v. What should you wear on a hot day, a black shirt or a white one?

Insulation & Thermal Resistance v. Insulators v. A material that does not easily conduct thermal energy or electricity v. Poor conductors with tightly bound valence electrons v. What is the particle difference between conductors and insulators? v. Aerogels v. The best artificial insulators v. Facet page 194

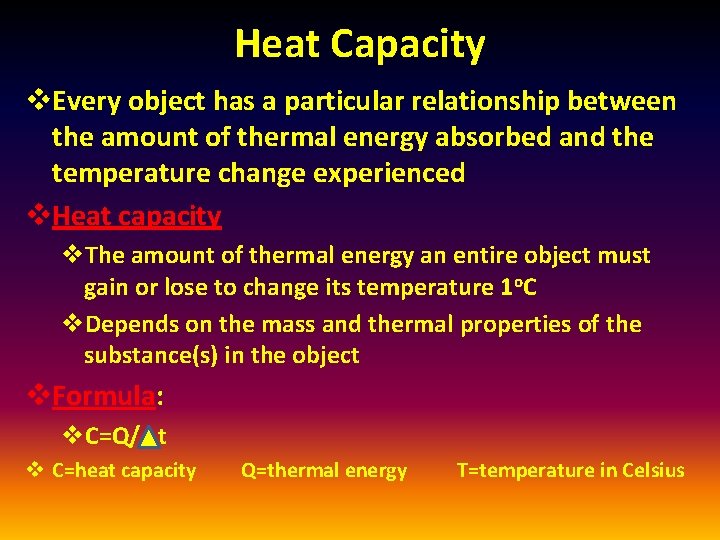
Heat Capacity v. Every object has a particular relationship between the amount of thermal energy absorbed and the temperature change experienced v. Heat capacity v. The amount of thermal energy an entire object must gain or lose to change its temperature 1 o. C v. Depends on the mass and thermal properties of the substance(s) in the object v. Formula: v. C=Q/ t v C=heat capacity Q=thermal energy T=temperature in Celsius

Specific Heat Capacity v. The amount of thermal energy 1 g of a substance must gain or lose to change its temperature 1 o. C v. Calorimeter v. A device that measure thermal energy transfer between objects contained in a chamber insulated from its surroundings v. Formula: v. Q=mcsp t v. Example Problem 9 -1 page 196


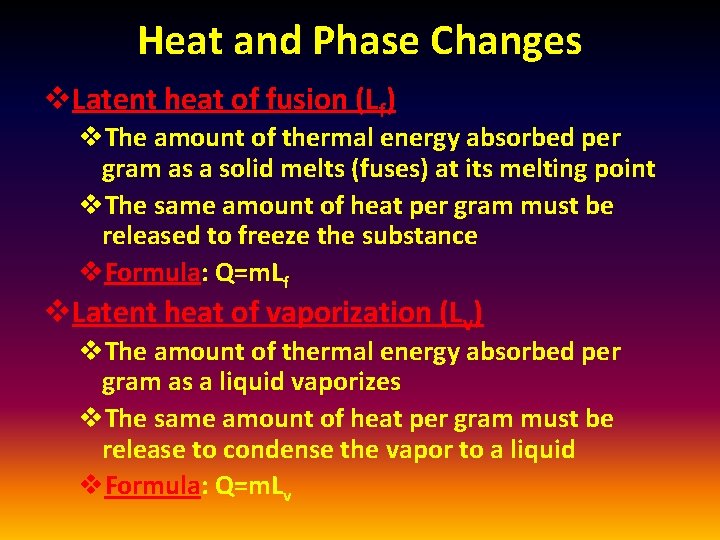
Heat and Phase Changes v. Latent heat of fusion (Lf) v. The amount of thermal energy absorbed per gram as a solid melts (fuses) at its melting point v. The same amount of heat per gram must be released to freeze the substance v. Formula: Q=m. Lf v. Latent heat of vaporization (Lv) v. The amount of thermal energy absorbed per gram as a liquid vaporizes v. The same amount of heat per gram must be release to condense the vapor to a liquid v. Formula: Q=m. Lv