Unit 2 lec 4 ATOMS Draw an initial




- Slides: 17

Unit 2 lec 4 - ATOMS! • Draw an initial model of an atom on your atom model paper. • This model should be what you think a model looks like. • It may be helpful to label parts of your atom.

Origin of the idea of atom… • GREECE – Democritus (460 -370 b. c. ): matter is made of tiny particles atomos

John Dalton’s Atomic Theory- 1803 Five notions about atoms… 1. All matter is made of atoms 2. All atoms of given element are identical 3. The atoms of one element are different from other elements 4. Atoms combine in whole ratios to make compounds: LAW OF CONSTANT COMPOSITION 5. In chemical reactions atoms are separated, combined, or rearranged. (but cannot be destroyed)

Atomic Structure • • Atoms are composed of 3 subatomic particles. Electron- J. J. Thompson and Millikan Proton- William Thompson and Rutherford Neutron- Rutherford, Bohr

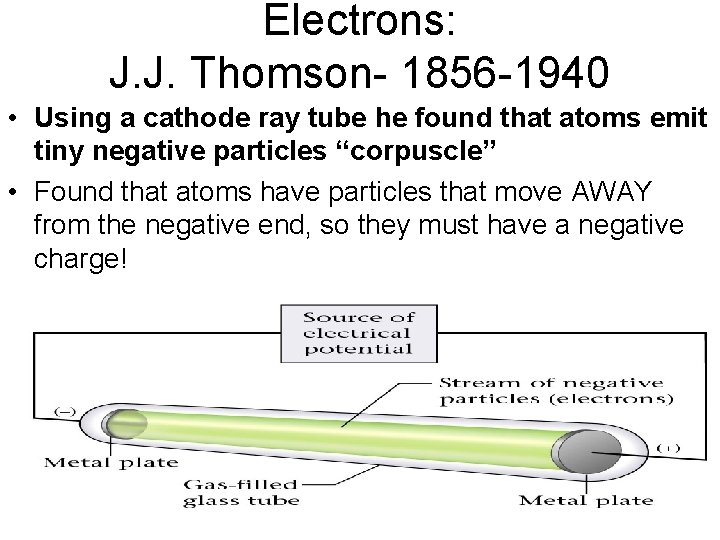
Electrons: J. J. Thomson- 1856 -1940 • Using a cathode ray tube he found that atoms emit tiny negative particles “corpuscle” • Found that atoms have particles that move AWAY from the negative end, so they must have a negative charge!


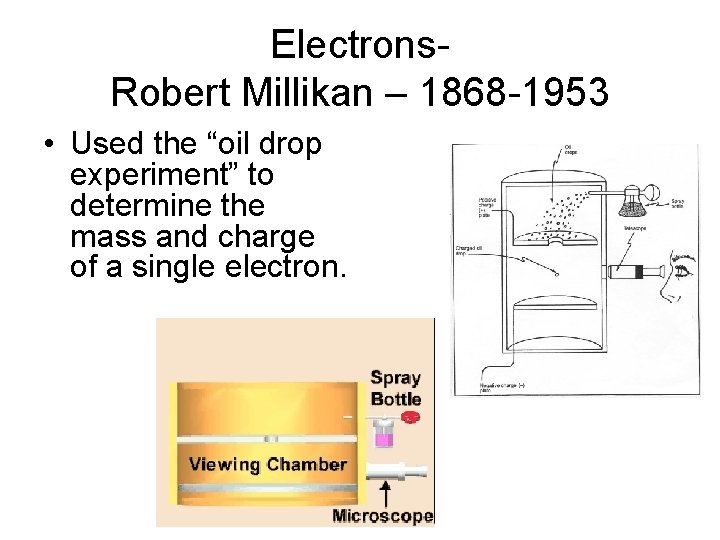
Electrons. Robert Millikan – 1868 -1953 • Used the “oil drop experiment” to determine the mass and charge of a single electron.


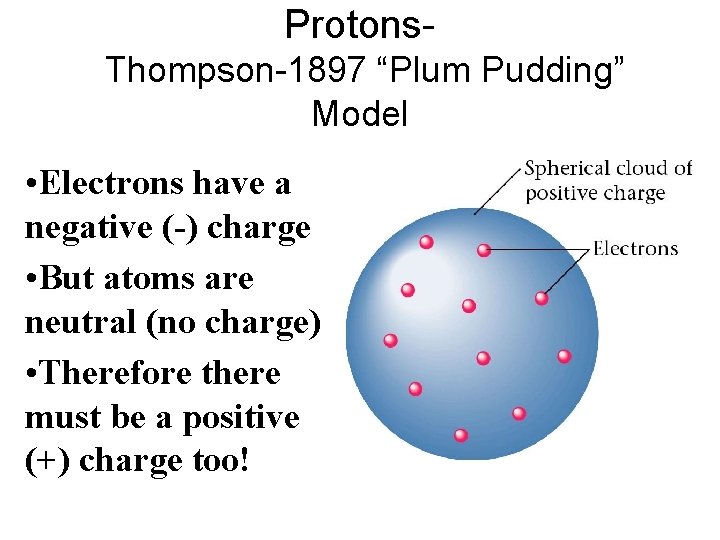
Protons. Thompson-1897 “Plum Pudding” Model • Electrons have a negative (-) charge • But atoms are neutral (no charge) • Therefore there must be a positive (+) charge too!

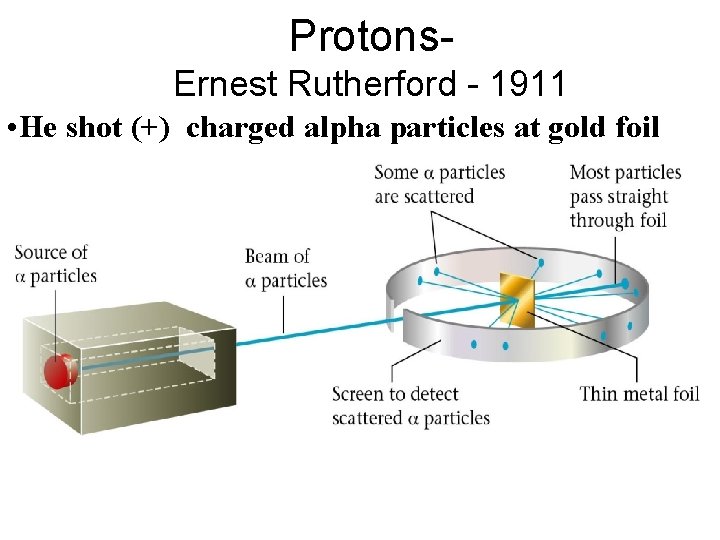
Protons. Ernest Rutherford - 1911 • He shot (+) charged alpha particles at gold foil


Protons. Ernest Rutherford - 1911 • Only a few positive particles were repelled • This disproved his hypothesis • There must be a VERY small positive central core (nucleus) , and the rest of the atom 99. 999% is empty space.

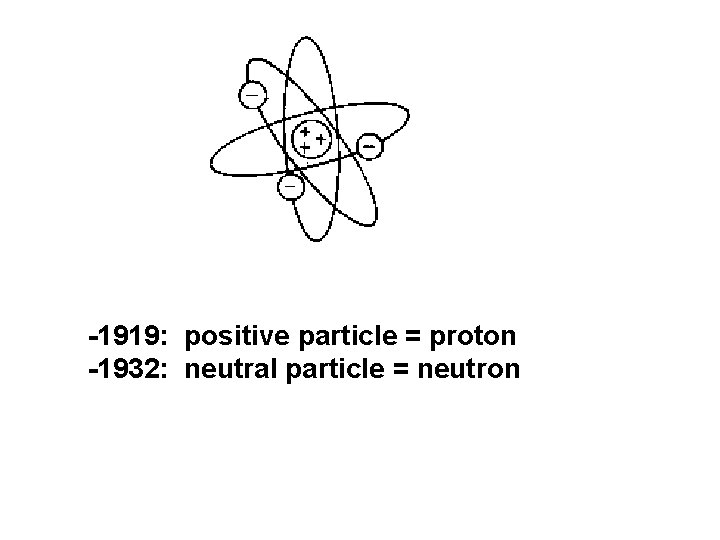
-1919: positive particle = proton -1932: neutral particle = neutron

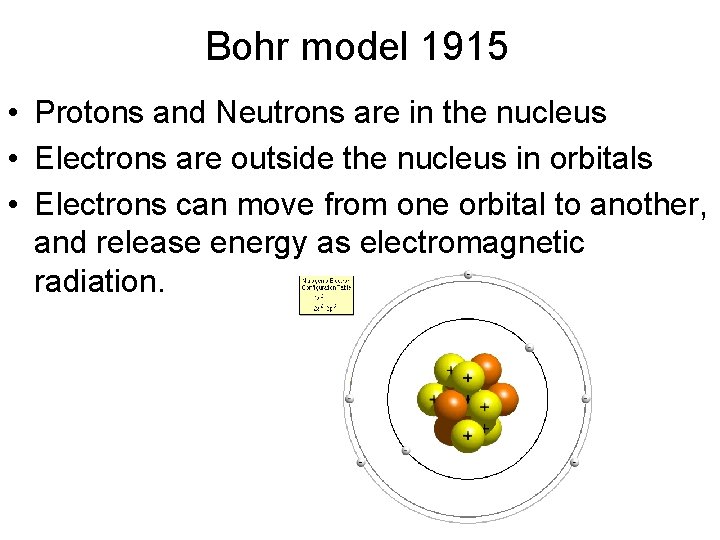
Bohr model 1915 • Protons and Neutrons are in the nucleus • Electrons are outside the nucleus in orbitals • Electrons can move from one orbital to another, and release energy as electromagnetic radiation.

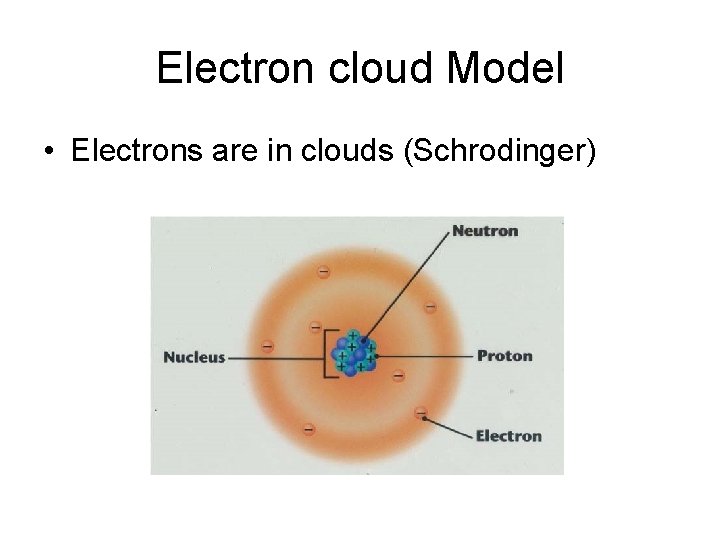
Electron cloud Model • Electrons are in clouds (Schrodinger)

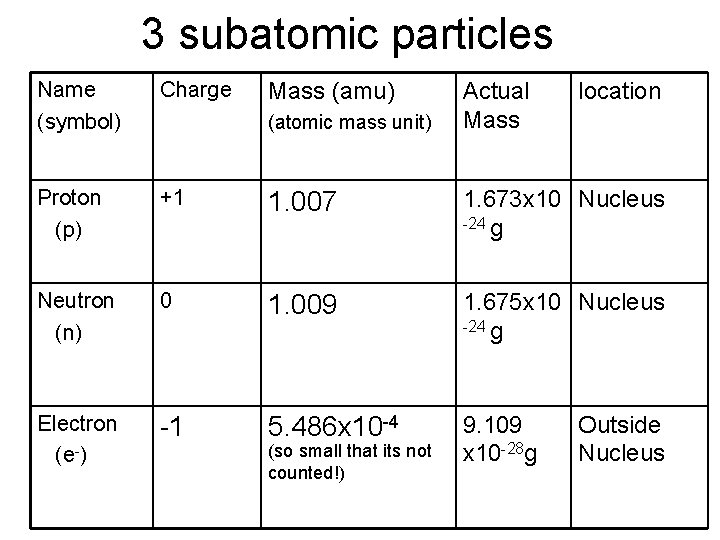
3 subatomic particles Name (symbol) Charge Proton (p) +1 1. 007 1. 673 x 10 Nucleus -24 g Neutron (n) 0 1. 009 1. 675 x 10 Nucleus -24 g Electron (e-) -1 5. 486 x 10 -4 9. 109 x 10 -28 g Mass (amu) (atomic mass unit) (so small that its not counted!) Actual Mass location Outside Nucleus

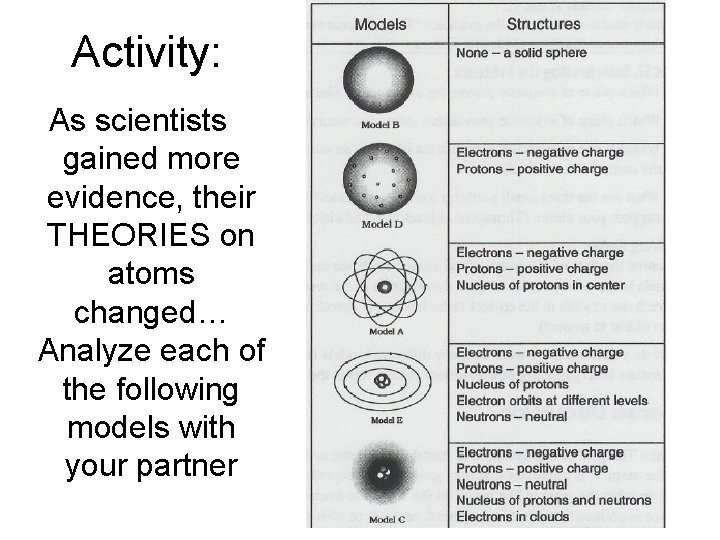
Activity: As scientists gained more evidence, their THEORIES on atoms changed… Analyze each of the following models with your partner