Unit 2 Introduction to Hydrocarbons Differences between organic

Unit 2 Introduction to Hydrocarbons

Differences between organic and inorganic compounds: 1. Organic compounds are mostly covalent molecules where most inorganics are ionic 2. Most organics don’t dissolve in water and most inorganics do 3. Organic compounds decompose on heating easier than inorganics 4. Organic reactions are much slower (min, hours, days) than inorganic reactions (seconds)

Fun Facts (Don’t have to Copy) More than 18 Million organic compounds [with 10, 000 new ones discovered each year] 1. 7 Million inorganic compounds so about 85% of compounds are organic
2 Reasons for the abundance of organic compounds: Carbon atoms bond to each other to form long chains(up to 200 carbons) Catenation – the ability of an element to bond to itself The same number of carbon atoms can rearrange to form different structures (isomers) Isomer – compounds with the same molecular formula but different structures

Isomer Example C 5 H 12 C -C-C-C- C -C-C-CC

How Carbon Bonds C ground state is 2 s 22 p 2 but bonds as *2 s 12 p 3 giving 4 sp 3 hybrid orbitals Hybrid orbitals – orbitals of equal energy formed by mixing orbitals of different energies Hybridization – the mixing of orbitals of different energies to give orbitals of equal energy
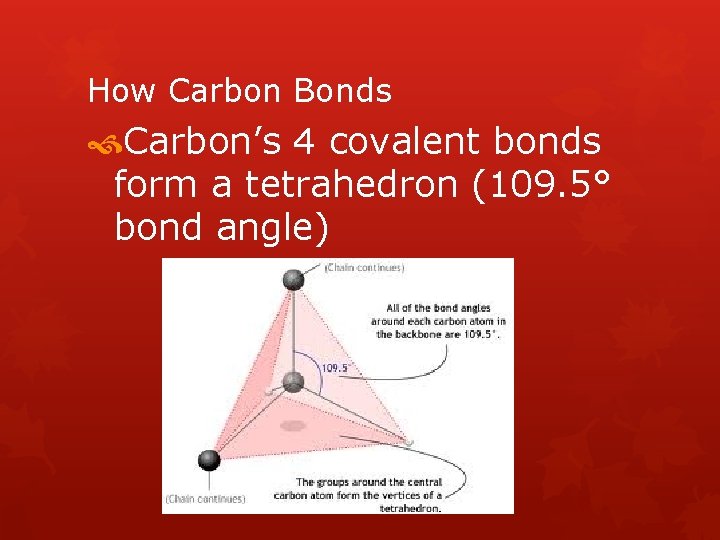
How Carbon Bonds Carbon’s 4 covalent bonds form a tetrahedron (109. 5° bond angle)

Hydrocarbons! Hydrocarbons – compounds containing only hydrogen and carbon Alkanes – hydrocarbons that have all C-C single bonds
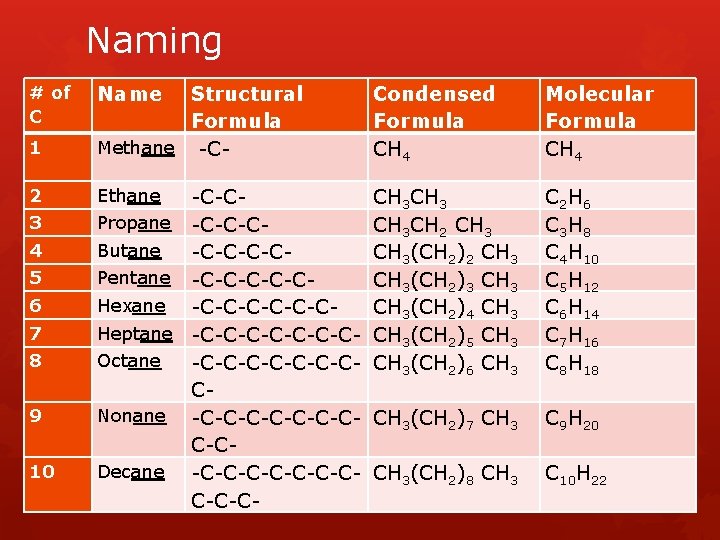
Naming # of C Name 1 Structural Formula Methane -C- Condensed Formula CH 4 Molecular Formula CH 4 2 Ethane CH 3 CH 2 CH 3(CH 2)3 CH 3(CH 2)4 CH 3(CH 2)5 CH 3(CH 2)6 CH 3 C 2 H 6 C 3 H 8 C 4 H 10 C 5 H 12 C 6 H 14 C 7 H 16 C 8 H 18 CH 3(CH 2)7 CH 3 C 9 H 20 CH 3(CH 2)8 CH 3 C 10 H 22 3 4 5 6 7 8 9 10 -C-CPropane -C-C-CButane -C-CPentane -C-C-CHexane -C-C-CHeptane -C-C-C-COctane -C-C-C-CCNonane -C-C-C-CC-CDecane -C-C-C-CC-C-C-

Naming *Know roots and endings* Each step, you add a CH 2 group Homologous Series – a series of compounds where each member differs from the next by a constant unit (CH 2) Members are called homologous Since alkanes are homologous – we can write a General Formula = Cn. H 2 n+2

Naming Alkanes are Saturated Hydrocarbons – hydrocarbons where each C has 4 single covalent bonds (no more atoms can be added)

Alkenes – hydrocarbons with one C=C double bond – sp 2 hybridization on the 2 C atoms in the double bond. Ethene C=C CH 2 C 2 H 4 Propene C=C-C CH 2 CHCH 3 C 3 H 6 Butene C=C-C-C Octene C=C-C-C-C- CH 2 CH 3 C 4 H 8 CH 2 CH(CH 2)5 CH 3 C 8 H 16
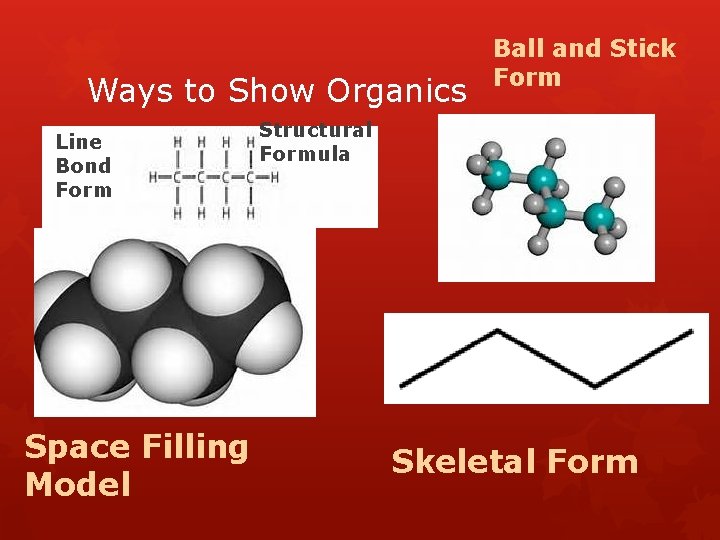
Ways to Show Organics Line Bond Form Space Filling Model Ball and Stick Form Structural Formula Skeletal Form

Alkenes Also a homologous series General Formula Cn. H 2 n Unsaturated hydrocarbons – have C-C multiple bonds which can be broken to add more atoms to the molecule H Ex: H C=C H H H + H 2 H H-C -C-H H H
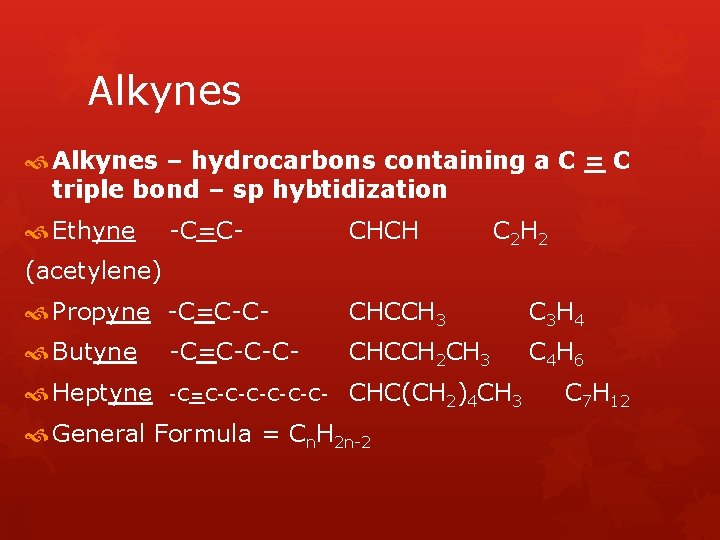
Alkynes – hydrocarbons containing a C = C triple bond – sp hybtidization Ethyne -C=C- CHCH C 2 H 2 (acetylene) Propyne -C=C-C- CHCCH 3 C 3 H 4 Butyne -C=C-C-C- CHCCH 2 CH 3 C 4 H 6 Heptyne -C=C-C-C- CHC(CH 2)4 CH 3 General Formula = Cn. H 2 n-2 C 7 H 12

Alkadienes – hydrocarbons containing two C=C double bonds Butadiene -C=C- Pentadiene -C=C-C- CH 2(CH)2 CH 2 C 4 H 6 CH 2(CH)3 CH 3 C 5 H 8 Heptadiene -C=C-C-C-CCH 2(CH)3(CH 2)2 CH 3 General Formula = Cn. H 2 n-2 C 7 H 12

Alkadienes 3 placements for the two double bonds Conjugated double bonds (most common) – two double bonds separated by one singe bond Isolated double bonds – two double bonds separated by more than one single bond Allenes – hydrocarbons that have two consecutive double bonds

The first 4 Series of hydrocarbons are Aliphatic Hydrocarbons Aliphatic hydrocarbons – hydrocarbon where carbon atoms bond together in open chains

Arenes Aromatic Hydrocarbons – hydrocarbons containing rings of 6 carbon atoms joined by alternating single and double bonds Simplest aromatic hydrocarbon = benzene
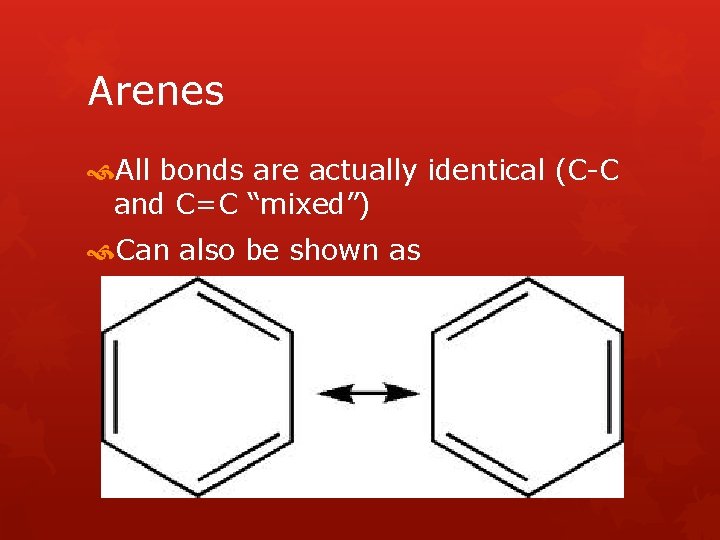
Arenes All bonds are actually identical (C-C and C=C “mixed”) Can also be shown as

Arenes We use The e-‘s are actually shared by all 6 carbons and move freely around the ring (delocalized) This makes benzene behave like saturated hydrocarbons

Resonance Compounds like these are resonance hybrids (compounds that can be represented by more than one Lewis structure) General Formula = Cn. H 1/2 n+3

Resonance examples

IUPAC Naming Rules 1. Name the longest chain (the parent chain) first. 2. Label the chain to give the lowest numbers to groups or bonds. Priority C=C then C=C You give the number for the carbon where the multiple bond begins. (Separate numbers and words with a hyphen, and numbers with a comma). 6 5 4 3 2 1 C-C-C-C=C-C C-C-C=C-C-C-C 2 –hexene 3 – heptyne

IUPAC Naming Rules 3. Give the numbers for any attached groups for the carbons they are attached to, a number for each attached group. Use the number with the groups name. [in front of “main” chain] a) If more than one of any group = di-, tri-, tetra -, penta-, hexa-, etc. b) Group Names: F = fluoro I = iodo Cl = chloro OH = hydroxo Br = bromo NO 2 = nitro

IUPAC Naming Rules c. If there is more than one group attached, the names are listed in alphabetical order (ignore prefixes) in front of the “main” chain d. If the numbers for the side groups are the same from either side of the chain, # from the side that gives the lowest # to the first group in the alpha order.

Summary Hydrocarbons (straight chains) Locate and name attached groups Locate multiple bonds (priority for numbering) Name base/parent chain

IUPAC Naming Rules 4. Branched chains Longest continuous chain containing any multiple bonds (if present) # to give multiple bonds lowest numbers (priority) Name side groups (alphabetical order)
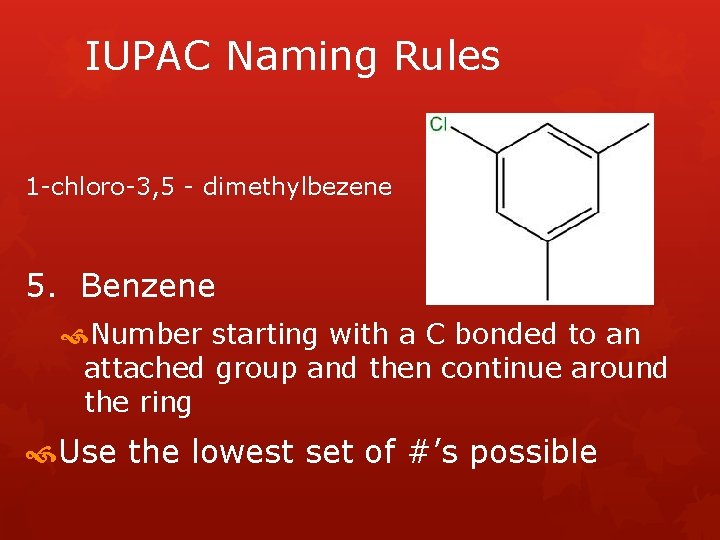
IUPAC Naming Rules 1 -chloro-3, 5 - dimethylbezene 5. Benzene Number starting with a C bonded to an attached group and then continue around the ring Use the lowest set of #’s possible
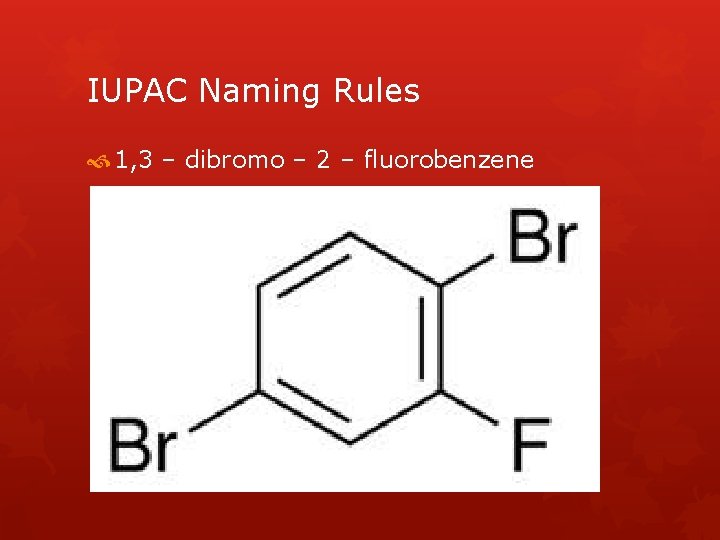
IUPAC Naming Rules 1, 3 – dibromo – 2 – fluorobenzene

IUPAC Naming Rules c. If there are just two of the same group attached, we can use the following terms to simplify the bonding positions Ortho = 1, 2 bonding position Meta = 1, 3 bonding position Para = 1, 4 bonding position
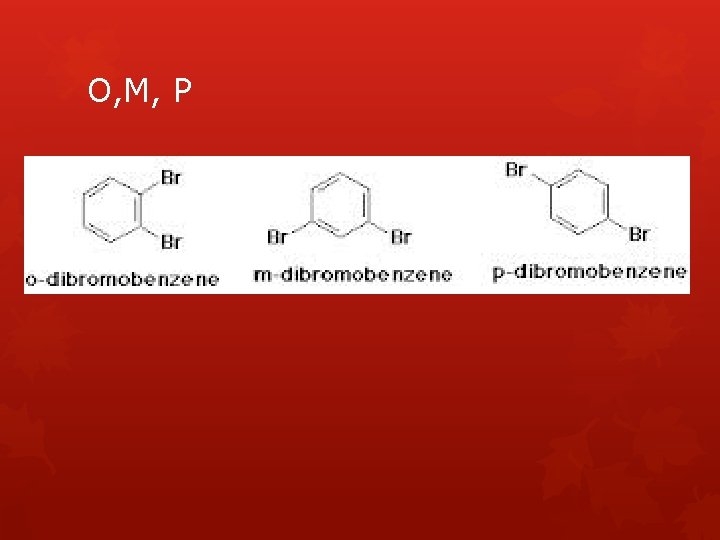
O, M, P

Isomer Practice Isomers – compounds having the same molecular formula but having different structures Example: C 5 H 11 Cl Draw all isomers by moving Cl (we are only going to use straight chains for C’s)

Isomer Practice Cl C–C–C Cl C –C – C – C Cl C–C–C

Isomer Practice C 4 H 8 Cl 2

Isomer Practice Try C 4 H 8 Cl. I, C 4 H 7 I 3, C 5 H 10 FBr, and C 4 H 7 F 2 Br
- Slides: 36