Unit 2 Formula Writing Naming Compounds Equations Stoichiometry























- Slides: 82

Unit 2: Formula Writing, Naming Compounds, Equations, & Stoichiometry

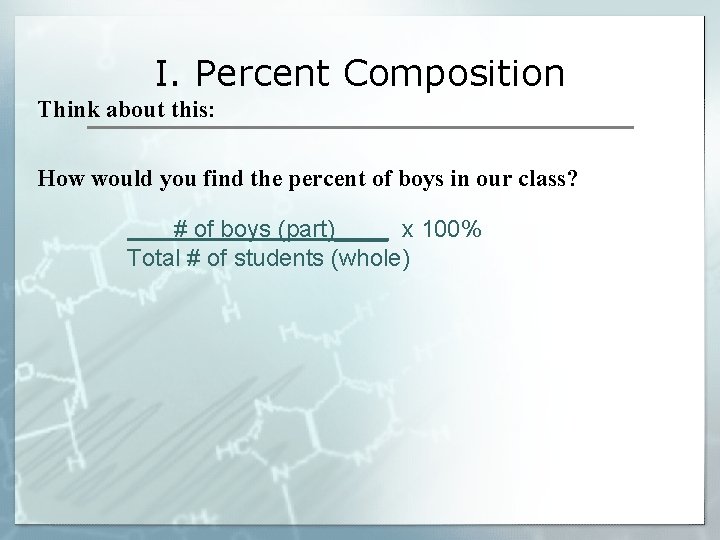
I. Percent Composition Think about this: How would you find the percent of boys in our class? # of boys (part)____ x 100% Total # of students (whole)

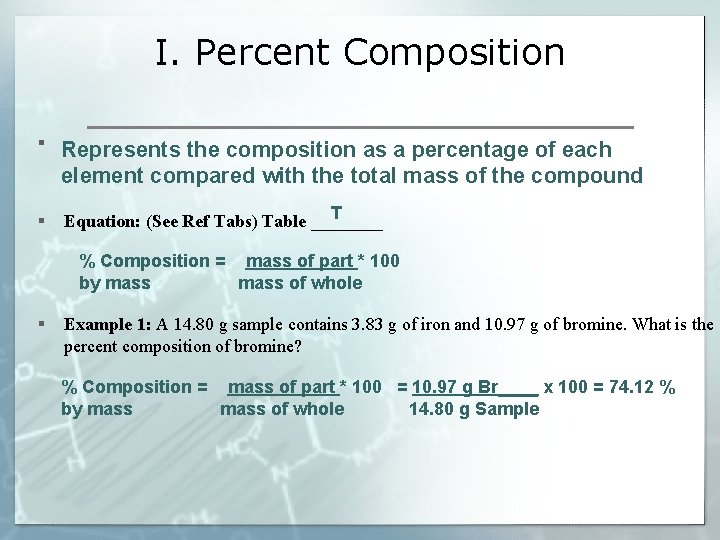
I. Percent Composition § Represents the composition as a percentage of each element compared with the total mass of the compound § T Equation: (See Ref Tabs) Table ____ % Composition = mass of part * 100 by mass of whole § Example 1: A 14. 80 g sample contains 3. 83 g of iron and 10. 97 g of bromine. What is the percent composition of bromine? % Composition = mass of part * 100 = 10. 97 g Br____ x 100 = 74. 12 % by mass of whole 14. 80 g Sample

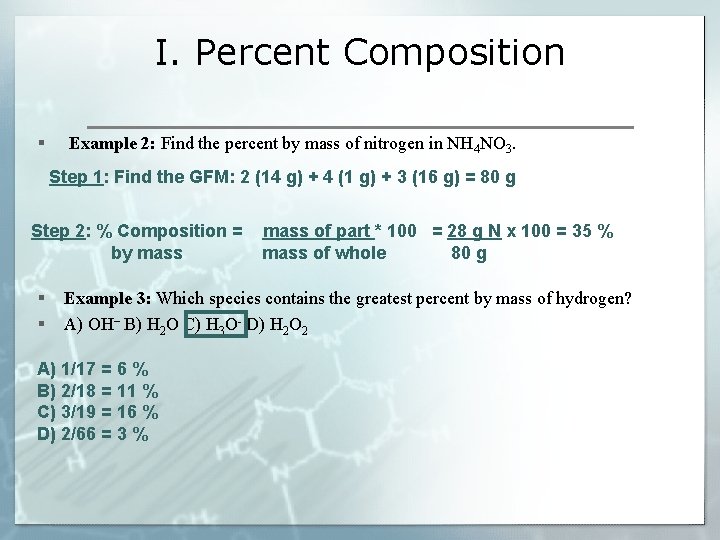
I. Percent Composition § Example 2: Find the percent by mass of nitrogen in NH 4 NO 3. Step 1: Find the GFM: 2 (14 g) + 4 (1 g) + 3 (16 g) = 80 g Step 2: % Composition = by mass § § mass of part * 100 = 28 g N x 100 = 35 % mass of whole 80 g Example 3: Which species contains the greatest percent by mass of hydrogen? A) OH– B) H 2 O C) H 3 O- D) H 2 O 2 A) 1/17 = 6 % B) 2/18 = 11 % C) 3/19 = 16 % D) 2/66 = 3 %

II. Percent Hydrate Think about this: A furry mole gets caught in a rain storm. His fur becomes wet and raises his mass to 125 g. He dries off and has a mass of 120 g. How would you find the percent of water that was on the wet mole? Difference in Masses of the Mole x 100% Total Mass of Wet Mole 5 g x 100% = 4% 125 g

II. Percent Hydrate § Hydrate: A compound that contains a specific amount of water molecules bound to its atoms § Anhydrate: A hydrate that has had the water driven off (evaporated) § The water is NOT bonded to the compound. It is attracted by a molecule-ion force of attraction. § Anhydrates can be used to absorb moisture in packaged electronic equipment and clothing. You have probably seen packages of sodium silicate (an anhydrate) used when buying a pair of shoes.

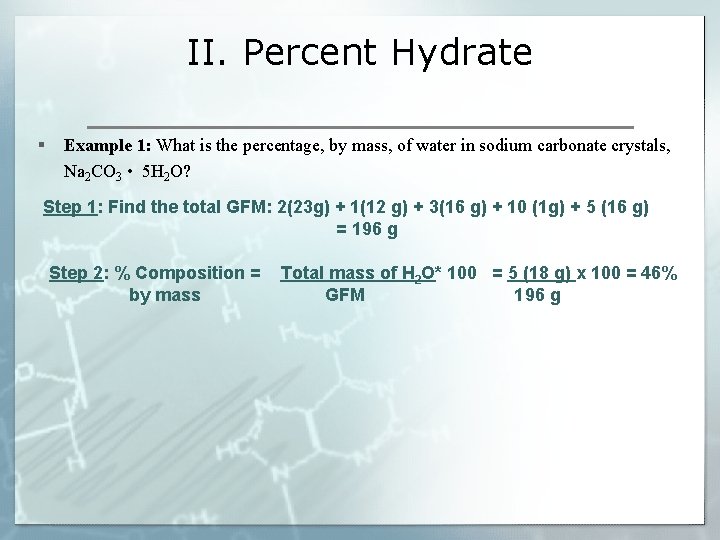
II. Percent Hydrate § Example 1: What is the percentage, by mass, of water in sodium carbonate crystals, Na 2 CO 3 • 5 H 2 O? Step 1: Find the total GFM: 2(23 g) + 1(12 g) + 3(16 g) + 10 (1 g) + 5 (16 g) = 196 g Step 2: % Composition = by mass Total mass of H 2 O* 100 = 5 (18 g) x 100 = 46% GFM 196 g

II. Percent Hydrate § Example 2: What is the percentage, by mass, of water in barium chloride crystals, Ba. Cl 2 • 2 H 2 O? % Composition = by mass § Total mass of H 2 O* 100 = 2 (18 g) x 100 = 15% GFM 243 g Example 3: Find the percent of water of hydration for Cu. SO 4 • 5 H 2 O. % Composition = by mass Total mass of H 2 O* 100 = 5 (18 g) x 100 = 36% GFM 250 g

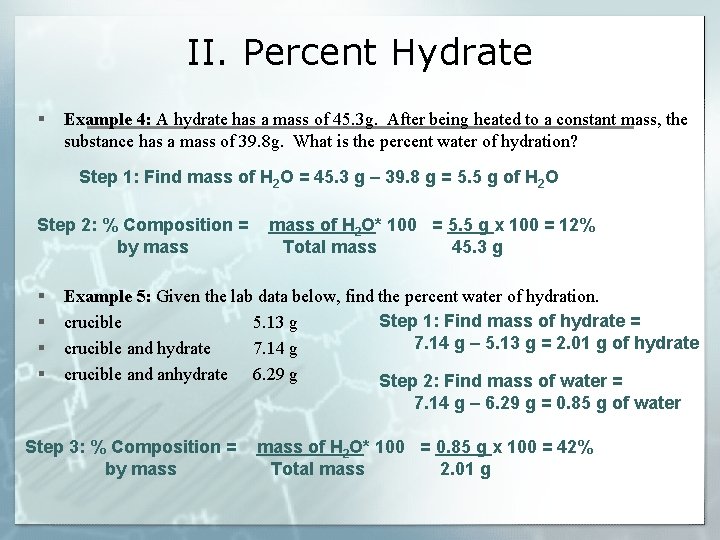
II. Percent Hydrate § Example 4: A hydrate has a mass of 45. 3 g. After being heated to a constant mass, the substance has a mass of 39. 8 g. What is the percent water of hydration? Step 1: Find mass of H 2 O = 45. 3 g – 39. 8 g = 5. 5 g of H 2 O Step 2: % Composition = by mass § § mass of H 2 O* 100 = 5. 5 g x 100 = 12% Total mass 45. 3 g Example 5: Given the lab data below, find the percent water of hydration. Step 1: Find mass of hydrate = crucible 5. 13 g 7. 14 g – 5. 13 g = 2. 01 g of hydrate crucible and hydrate 7. 14 g crucible and anhydrate 6. 29 g Step 2: Find mass of water = 7. 14 g – 6. 29 g = 0. 85 g of water Step 3: % Composition = by mass of H 2 O* 100 = 0. 85 g x 100 = 42% Total mass 2. 01 g

II. Percent Hydrate (Practice) § Complete problems 4 and 6 from pg 15 (if you finish early, move on to problem 2 and 7)

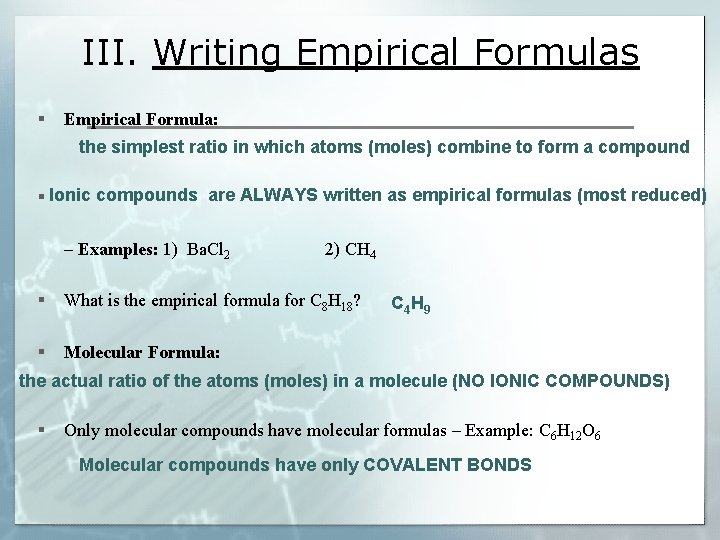
III. Writing Empirical Formulas § Empirical Formula: the simplest ratio in which atoms (moles) combine to form a compounds are ALWAYS written as empirical formulas (most reduced) § Ionic – Examples: 1) Ba. Cl 2 2) CH 4 § What is the empirical formula for C 8 H 18? § Molecular Formula: C 4 H 9 the actual ratio of the atoms (moles) in a molecule (NO IONIC COMPOUNDS) § Only molecular compounds have molecular formulas – Example: C 6 H 12 O 6 Molecular compounds have only COVALENT BONDS

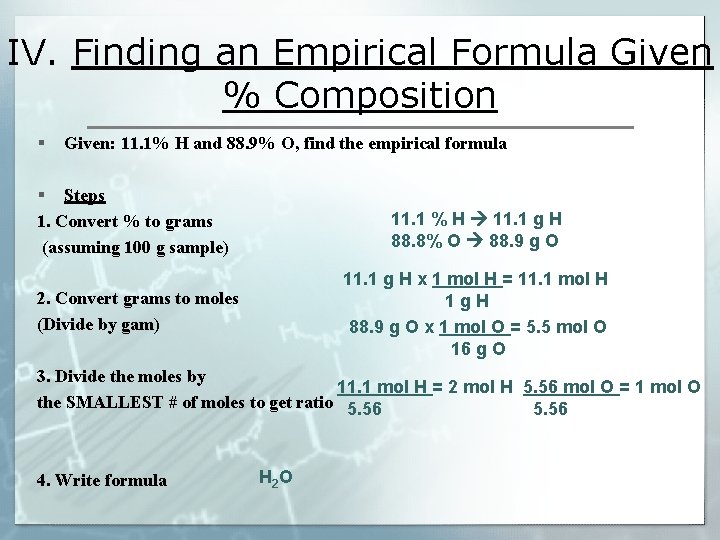
IV. Finding an Empirical Formula Given % Composition § Given: 11. 1% H and 88. 9% O, find the empirical formula § Steps 1. Convert % to grams (assuming 100 g sample) 11. 1 % H 11. 1 g H 88. 8% O 88. 9 g O 2. Convert grams to moles (Divide by gam) 11. 1 g H x 1 mol H = 11. 1 mol H 1 g. H 88. 9 g O x 1 mol O = 5. 5 mol O 16 g O 3. Divide the moles by 11. 1 mol H = 2 mol H 5. 56 mol O = 1 mol O the SMALLEST # of moles to get ratio 5. 56 4. Write formula H 2 O

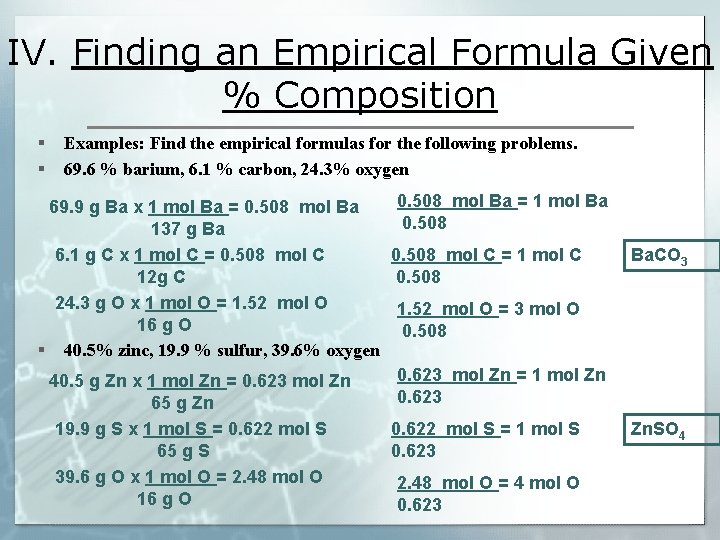
IV. Finding an Empirical Formula Given % Composition § § Examples: Find the empirical formulas for the following problems. 69. 6 % barium, 6. 1 % carbon, 24. 3% oxygen 69. 9 g Ba x 1 mol Ba = 0. 508 mol Ba 137 g Ba 6. 1 g C x 1 mol C = 0. 508 mol C 12 g C 24. 3 g O x 1 mol O = 1. 52 mol O 16 g O § 40. 5% zinc, 19. 9 % sulfur, 39. 6% oxygen 40. 5 g Zn x 1 mol Zn = 0. 623 mol Zn 65 g Zn 19. 9 g S x 1 mol S = 0. 622 mol S 65 g S 39. 6 g O x 1 mol O = 2. 48 mol O 16 g O 0. 508 mol Ba = 1 mol Ba 0. 508 mol C = 1 mol C 0. 508 Ba. CO 3 1. 52 mol O = 3 mol O 0. 508 0. 623 mol Zn = 1 mol Zn 0. 623 0. 622 mol S = 1 mol S 0. 623 2. 48 mol O = 4 mol O 0. 623 Zn. SO 4

IV. Finding an Empirical Formula Given % Composition § If you don’t end up within a tenth of a whole number, multiply both numbers by 2 to get whole number ratio § Example - A compound of zinc and phosphorous, when analyzed, showed 76. 0% Zn and 24. 0% P by mass. Calculate the simplest formula for the compound. 76. 0 g Zn x 1 mol Zn = 1. 17 mol Zn 65 g Zn 24. 0 g P x 1 mol P = 0. 774 mol P 31 g P 1. 17 mol Zn= 1. 5 mol Zn * 2 = 3 Zn 0. 774 mol P = 1 mol P * 2 = 2 P 0. 774 Zn 3 P 2

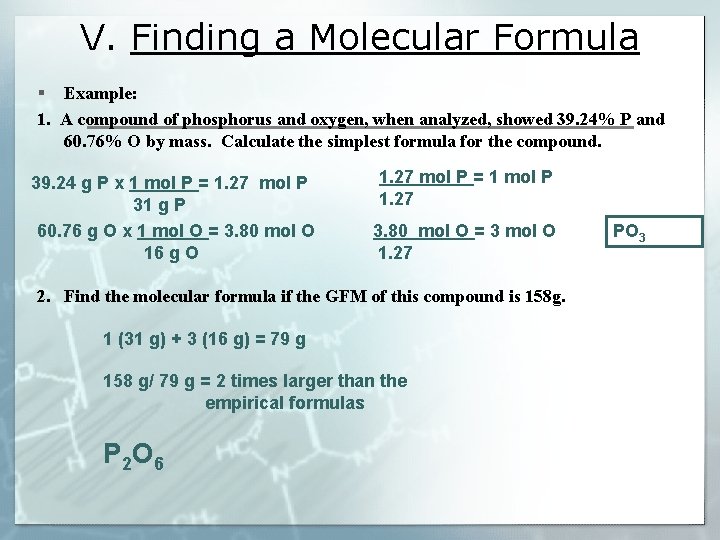
V. Finding a Molecular Formula § What is the molecular formula of a compound that has a GFM of 92 g and an empirical formula of NO 2? § Steps 1. Find the empirical formula NO 2 2. Find the mass of the empirical 1 (14 g) + 2 (16 g) = 46 g formula 3. Find how many times larger the 92 g/ 46 g = 2 times larger than the GFM is than the empirical formulas and multiply the subscripts by that number. N 2 O 4

V. Finding a Molecular Formula § Example: 1. A compound of phosphorus and oxygen, when analyzed, showed 39. 24% P and 60. 76% O by mass. Calculate the simplest formula for the compound. 39. 24 g P x 1 mol P = 1. 27 mol P 31 g P 60. 76 g O x 1 mol O = 3. 80 mol O 16 g O 1. 27 mol P = 1 mol P 1. 27 3. 80 mol O = 3 mol O 1. 27 2. Find the molecular formula if the GFM of this compound is 158 g. 1 (31 g) + 3 (16 g) = 79 g 158 g/ 79 g = 2 times larger than the empirical formulas P 2 O 6 PO 3

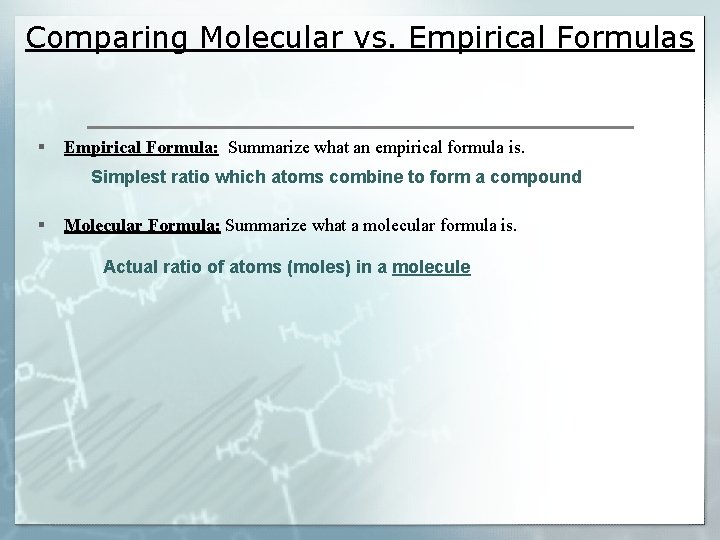
Comparing Molecular vs. Empirical Formulas § Empirical Formula: Summarize what an empirical formula is. Simplest ratio which atoms combine to form a compound § Molecular Formula: Summarize what a molecular formula is. Actual ratio of atoms (moles) in a molecule

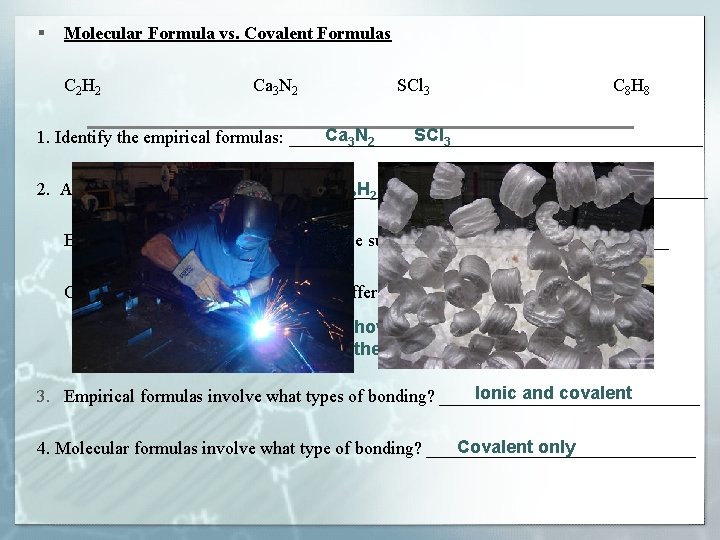
§ Molecular Formula vs. Covalent Formulas C 2 H 2 Ca 3 N 2 SCl 3 C 8 H 8 Ca 3 N 2 SCl 3 1. Identify the empirical formulas: _______________________ 2. A) Identify the molecular formulas: ______________________ C 2 H 2 C 8 H 8 CH B) What is the empirical formula of these substances? ____________ C) Why would these substances have different chemical properties? The molecular formula indicates how many atoms are bonded. The way and number of bonds affect the properties. Ionic and covalent 3. Empirical formulas involve what types of bonding? _______________ Covalent only 4. Molecular formulas involve what type of bonding? _______________

§ Practice Problems: Show your work on the whiteboard and call Mrs. Pelc over once your group has completed the entire problem. Be sure to show all work for each problem. 1. A compound has an empirical formula Al 2 O 3. A)Why does this compound not have a molecular formula? B) Calculate the percent composition of each element. 2. A substance has a formula of C 8 H 18. A) What is the empirical formula of this substance? B) Using the empirical formula, calculate the percent composition of each element? 3. A substance contains 30. 4% N and 69. 6% O. A) Calculate the empirical formula of the compound (show all work). B) If the compound has a gram formula mass of 92 g, what is the molecular formula of the substance? 4. A hydrate consists of 19. 17 % Na, 13. 33 % S, and 67. 50 % H 2 O. A) Calculate the empirical formula of the hydrate (show all work). B) Why does this hydrate not have a molecular formula?

VI. Naming Compounds Metals: positive ions (cations) lose e- and form _________ when bonding • Atoms that ______ Alloy: Mixture of metals by melting them together Properties of Metals: 1. Low ionization energy and electronegativity 2. Good conductors of heat and electricity 3. Exhibit metallic luster (shine) 4. Malleability (can be pounded into thin sheets) 5. Ductility (can be pulled into thin wires) 6. More than 2/3 of the elements are metals

High densities. 7. _____ liquid at room Mercury (Hg) is a metal which is a ______ 8. ______ temperature. Fr (francium) 9. Most active metal: _______

Nonmetals: • atoms gain e- and form negative ions (anions) when bonding • Properties of Nonmetals: 1. High ionization energy and electronegativity 2. Poor conductors of heat and electricity 3. Brittle and hard 4. Low densities F (Fluorine) (top right corner) 5. Most active nonmetal: ____ Graphite is an allotrope of carbon. It is used in pencils (brittle, soft, and low density)

Metalloids • Atoms that gain or lose e- and form ions when bonding • Have properties of both metals and nonmetals • Can be located using the “staircase” (see periodic table) • Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium

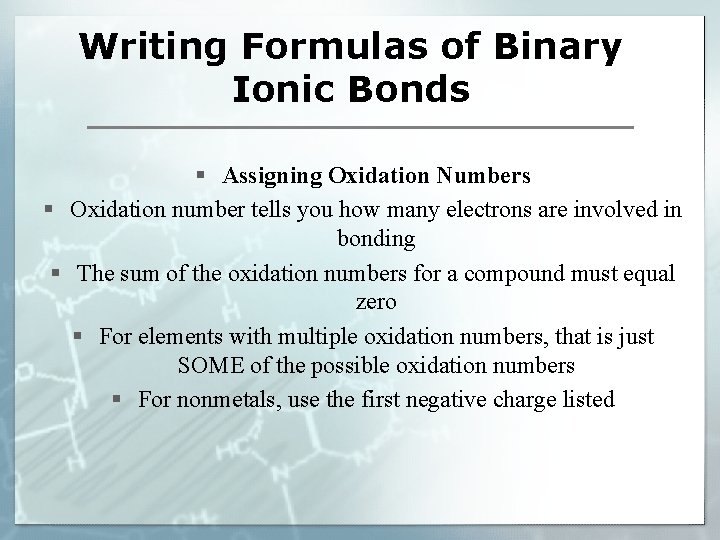
Writing Formulas of Binary Ionic Bonds § Assigning Oxidation Numbers § Oxidation number tells you how many electrons are involved in bonding § The sum of the oxidation numbers for a compound must equal zero § For elements with multiple oxidation numbers, that is just SOME of the possible oxidation numbers § For nonmetals, use the first negative charge listed

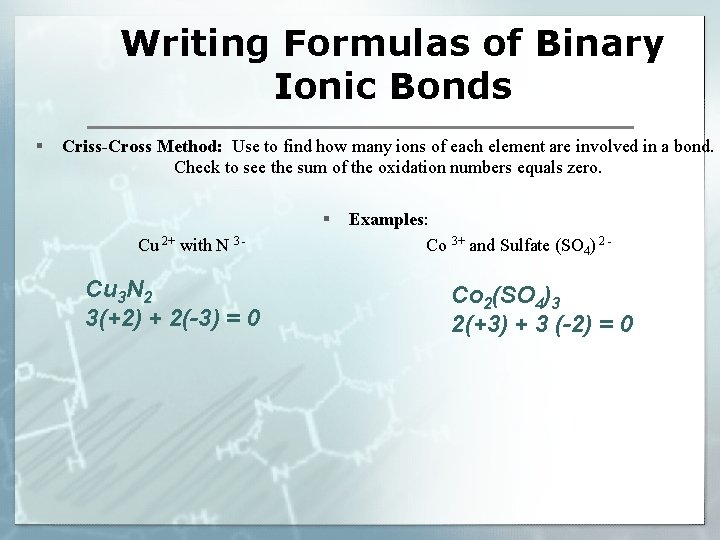
Writing Formulas of Binary Ionic Bonds § Criss-Cross Method: Use to find how many ions of each element are involved in a bond. Check to see the sum of the oxidation numbers equals zero. § Cu 2+ with N 3 - Cu 3 N 2 3(+2) + 2(-3) = 0 Examples: Co 3+ and Sulfate (SO 4) 2 - Co 2(SO 4)3 2(+3) + 3 (-2) = 0

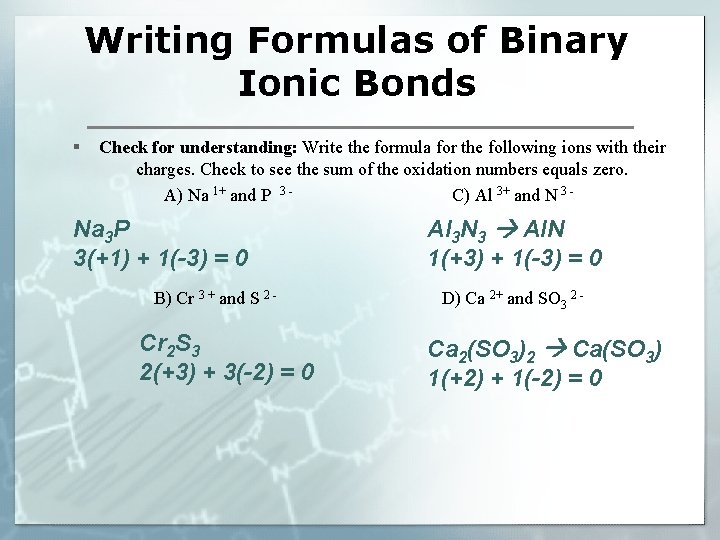
Writing Formulas of Binary Ionic Bonds § Check for understanding: Write the formula for the following ions with their charges. Check to see the sum of the oxidation numbers equals zero. A) Na 1+ and P 3 C) Al 3+ and N 3 - Na 3 P 3(+1) + 1(-3) = 0 B) Cr 3 + and S 2 - Cr 2 S 3 2(+3) + 3(-2) = 0 Al 3 N 3 Al. N 1(+3) + 1(-3) = 0 D) Ca 2+ and SO 3 2 - Ca 2(SO 3)2 Ca(SO 3) 1(+2) + 1(-2) = 0

- Naming Formulas of Binary Ionic Bonds § Nonmetal’s names change when forming ionic bonds Nitrogen Nitride Phosphorus Phosphide Sulfur Sulfide Oxygen Oxide Chlorine Chloride Fluorine Fluoride Bromine Bromide Iodine Iodide Carbon Carbide Hydrogen Hydride

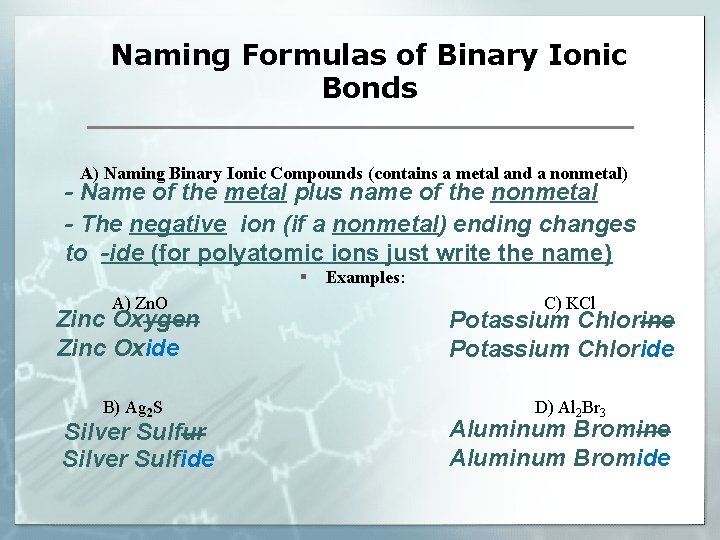
Naming Formulas of Binary Ionic Bonds A) Naming Binary Ionic Compounds (contains a metal and a nonmetal) - Name of the metal plus name of the nonmetal - The negative ion (if a nonmetal) ending changes to -ide (for polyatomic ions just write the name) § Examples: A) Zn. O Zinc Oxygen Zinc Oxide B) Ag 2 S Silver Sulfur Silver Sulfide C) KCl Potassium Chlorine Potassium Chloride D) Al 2 Br 3 Aluminum Bromine Aluminum Bromide

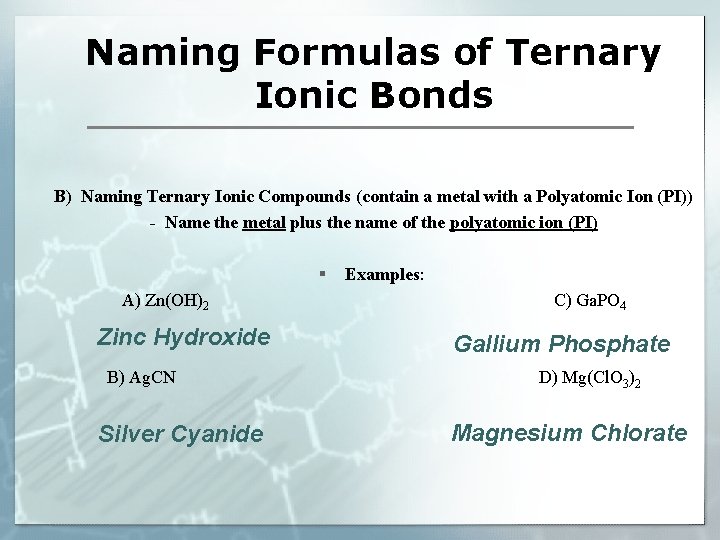
Naming Formulas of Ternary Ionic Bonds B) Naming Ternary Ionic Compounds (contain a metal with a Polyatomic Ion (PI)) - Name the metal plus the name of the polyatomic ion (PI) § Examples: A) Zn(OH)2 Zinc Hydroxide B) Ag. CN Silver Cyanide C) Ga. PO 4 Gallium Phosphate D) Mg(Cl. O 3)2 Magnesium Chlorate

Naming Formulas of Ternary Ionic Bonds C) Naming Ionic Compounds with Roman Numerals (contain a metal with multiple oxidation numbers) - Use the reverse criss-cross method to identify the charge of the ions. - If the positive ion has more than one listed charge (transition metals), use a Roman numeral after the metal’s name to indicate the charge (you do NOT use a Roman numeral for the nonmetal/PI) - If there is a 1 -1 ratio between the metal and negative ion, the charge of the metal will have the same charge of the negative ion (nonmetal/polyatomic ion)

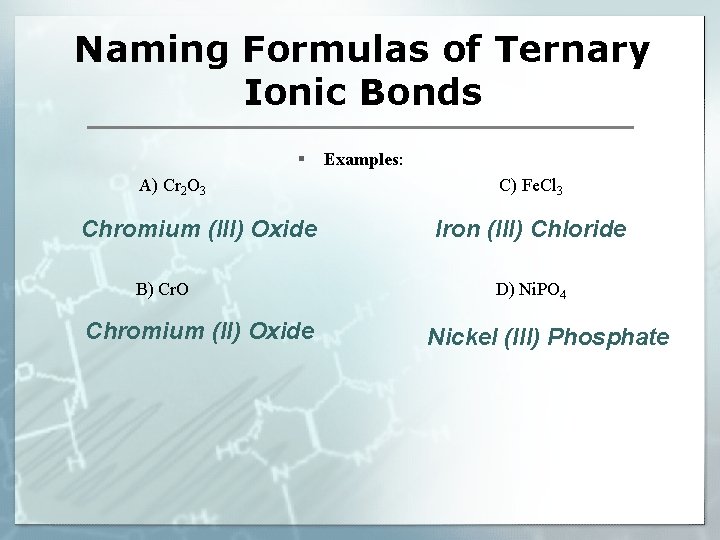
Naming Formulas of Ternary Ionic Bonds § A) Cr 2 O 3 Chromium (III) Oxide B) Cr. O Chromium (II) Oxide Examples: C) Fe. Cl 3 Iron (III) Chloride D) Ni. PO 4 Nickel (III) Phosphate

Writing Formulas of Ionic Bonds A) Writing Binary Ionic Compounds (contain a metal and nonmetal) - Write the symbols for the metal and nonmetal. - Look up the charges (oxidation numbers) for each of the ions. For the nonmetal, it is the first negative number listed. - Criss-cross the charges to get the number of ions used for each element and drop the +/- Reduce to the smallest ratio, if you can.

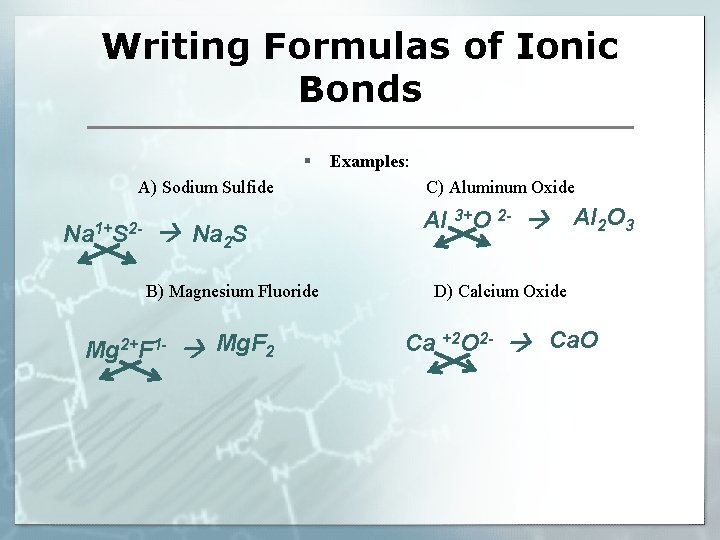
Writing Formulas of Ionic Bonds § A) Sodium Sulfide Na 1+S 2 - Na 2 S B) Magnesium Fluoride Mg 2+F 1 - Mg. F 2 Examples: C) Aluminum Oxide Al 3+O 2 - Al 2 O 3 D) Calcium Oxide Ca +2 O 2 - Ca. O

Writing Formulas of Ionic Bonds B) Writing Ternary Ionic Compounds (contain a metal with a PI ) - Write the symbols for the metal and PI. - Look up the charges (oxidation numbers) for each of the ions. - Criss-cross the charges to get the number of ions used for each element/PI and drop the +/- Reduce to the smallest ratio, if you can.

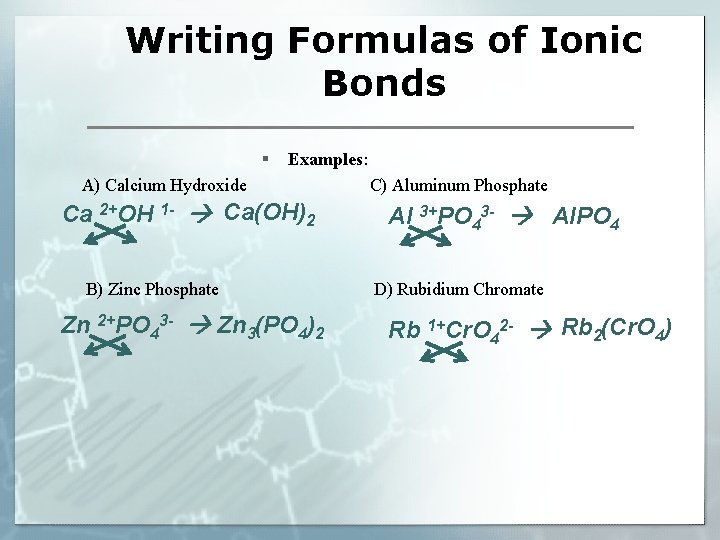
Writing Formulas of Ionic Bonds § Examples: A) Calcium Hydroxide Ca 2+OH 1 - Ca(OH)2 B) Zinc Phosphate Zn 2+PO 43 - Zn 3(PO 4)2 C) Aluminum Phosphate Al 3+PO 43 - Al. PO 4 D) Rubidium Chromate Rb 1+Cr. O 42 - Rb 2(Cr. O 4)

Writing Formulas of Ionic Bonds C) Writing Ionic Compounds with Roman Numerals (contain a metal with multiple oxidation numbers) - Write the symbols for the metal and nonmetal/PI. - If a Roman numeral is in the name, it represents the charge of the METAL. For the nonmetal, use the first negative charge listed. - Criss-cross the charges to get the number of ions used for each element/PI and drop the +/- Reduce to the smallest ratio, if you can.

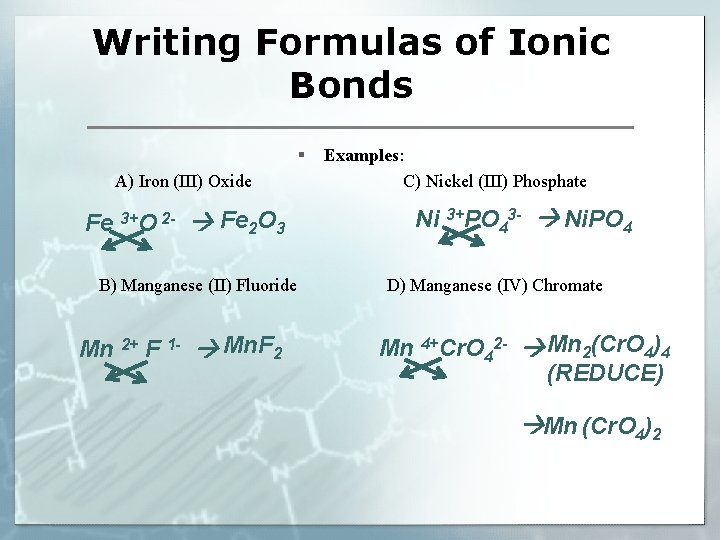
Writing Formulas of Ionic Bonds § A) Iron (III) Oxide Fe 3+O 2 - Fe 2 O 3 B) Manganese (II) Fluoride Mn 2+ F 1 - Mn. F 2 Examples: C) Nickel (III) Phosphate 3+PO 3 - Ni. PO Ni 4 4 D) Manganese (IV) Chromate Mn 4+Cr. O 42 - Mn 2(Cr. O 4)4 (REDUCE) Mn (Cr. O 4)2

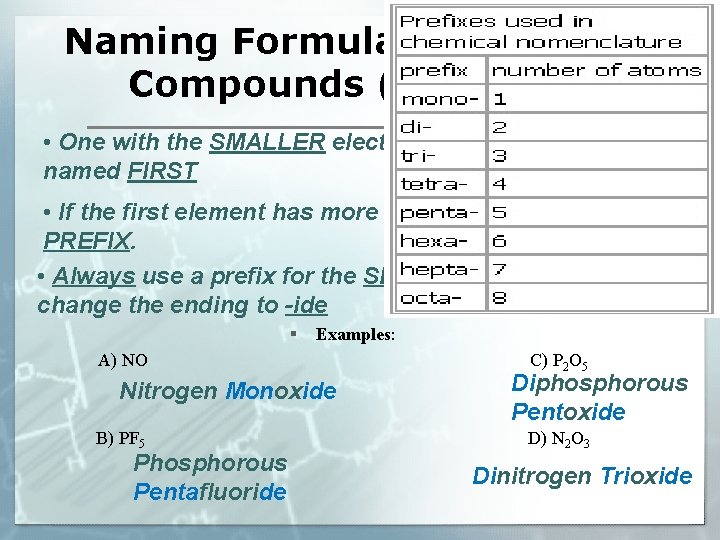
Naming Formulas of Molecular Compounds (Molecules) • One with the SMALLER electronegativity gets named FIRST • If the first element has more than one atom, use a PREFIX. • Always use a prefix for the SECOND element and change the ending to -ide § Examples: A) NO Nitrogen Monoxide B) PF 5 Phosphorous Pentafluoride C) P 2 O 5 Diphosphorous Pentoxide D) N 2 O 3 Dinitrogen Trioxide

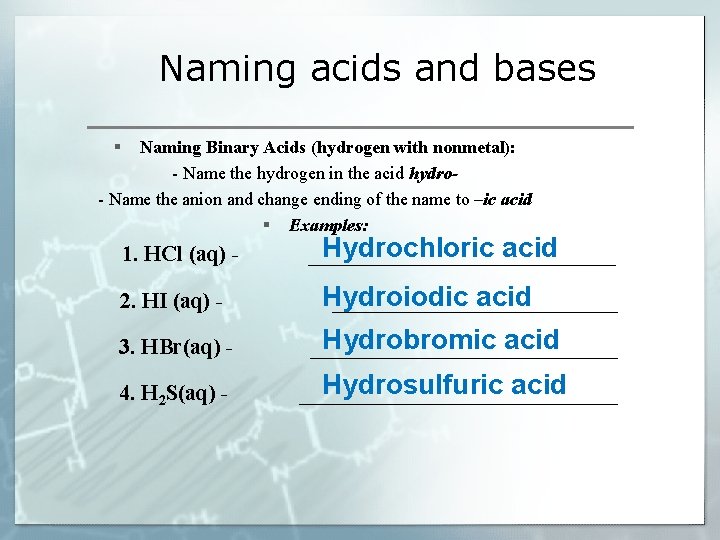
Naming acids and bases § Naming Binary Acids (hydrogen with nonmetal): - Name the hydrogen in the acid hydro- Name the anion and change ending of the name to –ic acid § Examples: 1. HCl (aq) - Hydrochloric acid ______________ Hydroiodic acid 2. HI (aq) - _____________ 3. HBr(aq) - Hydrobromic acid ______________ 4. H 2 S(aq) - Hydrosulfuric acid _______________

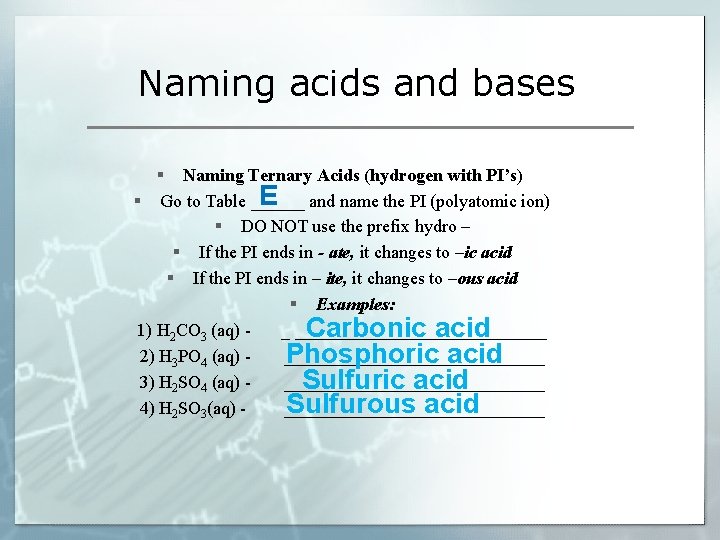
Naming acids and bases § Naming Ternary Acids (hydrogen with PI’s) E § Go to Table ______ and name the PI (polyatomic ion) § DO NOT use the prefix hydro – § If the PI ends in - ate, it changes to –ic acid § If the PI ends in – ite, it changes to –ous acid § Examples: 1) H 2 CO 3 (aq) _ ______________ Carbonic acid 2) H 3 PO 4 (aq) _______________ Phosphoric acid 3) H 2 SO 4 (aq) _______________ Sulfuric acid Sulfurous acid 4) H 2 SO 3(aq) _______________

Naming acids and bases § Naming Bases: IONIC COMPOUNDS - Bases are all __________, therefore, they are named just like ________ § Name 1 st element § Use roman numeral if the metal has more than one charge listed HYDROXIDE § 2 nd half of the name will typically be ________ § Examples: Sodium hydroxide 1. Na. OH (aq) __________

Naming acids and bases § Naming Bases (continued): 2. Ca(OH)2 (aq) - Calcium _______________ hydroxide 3. Fe(OH)3 (aq) - Iron (III) hydroxide _______________ (II) hydroxide 4. Co(OH)2 (aq) - Cobalt _______________

Naming Formulas of Ternary Molecular Compounds (Molecules with PI) 1. A polyatomic ion contains two or more elements bonded together charge with a _____. See Table E. metal 2. Name the _____ and then the polyatomic ion. Practice Naming Compounds with Polyatomic Ions 1. Name the following. Use Roman Numeral when necessary. sodium nitrate ammonium chloride __________ NH 4 Cl __________ Na. NO 3 __________ Ca. CO calcium carbonate iron (III) chlorate 3 __________ Fe(Cl. O 3 )3 magnesium hydrogen __________ Mg(HCO 3)2 __________ Zn. SO zinc sulfate 4 carbonate 2. Write formulas for the following. Balance the charges of the metal and polyatomic ion. K 2 C 2 O 4 Au(Cl. O 3)3 ________ potassium oxalate ________ gold (III) chlorate Na 2 S 2 O 3 Cu(OH)2 ________ sodium thiosulfate _______copper (II) hydroxide

Unit 2: Balancing Chemical Equations

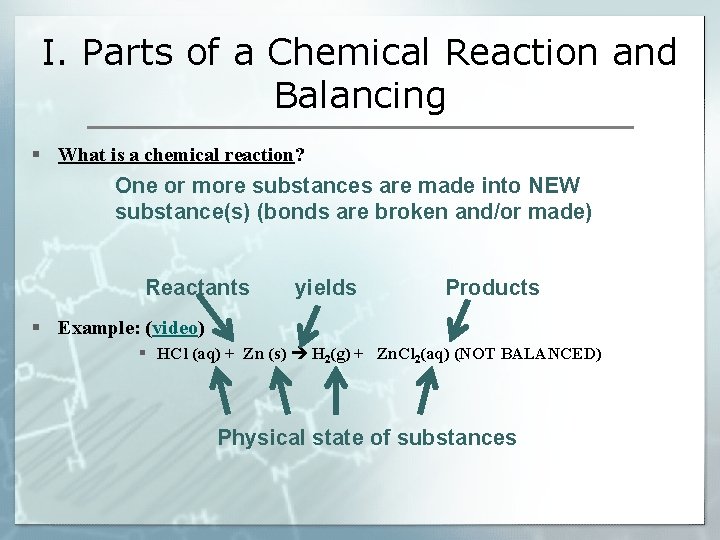
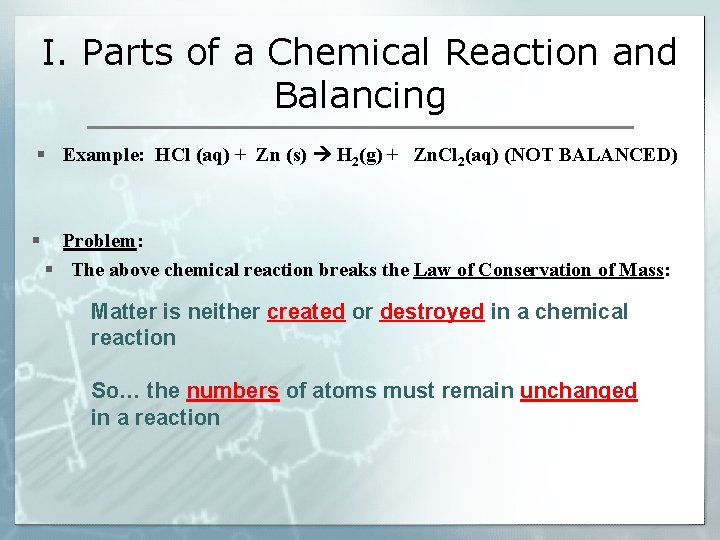
I. Parts of a Chemical Reaction and Balancing § What is a chemical reaction? One or more substances are made into NEW substance(s) (bonds are broken and/or made) Reactants yields Products § Example: (video) § HCl (aq) + Zn (s) H 2(g) + Zn. Cl 2(aq) (NOT BALANCED) Physical state of substances

I. Parts of a Chemical Reaction and Balancing § Example: HCl (aq) + Zn (s) H 2(g) + Zn. Cl 2(aq) (NOT BALANCED) § Problem: § The above chemical reaction breaks the Law of Conservation of Mass: Matter is neither created or destroyed in a chemical reaction So… the numbers of atoms must remain unchanged in a reaction

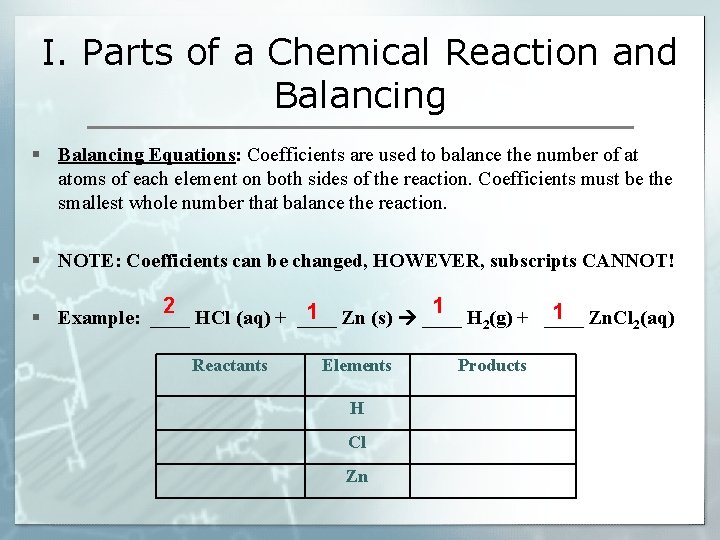
I. Parts of a Chemical Reaction and Balancing § Balancing Equations: Coefficients are used to balance the number of at atoms of each element on both sides of the reaction. Coefficients must be the smallest whole number that balance the reaction. § NOTE: Coefficients can be changed, HOWEVER, subscripts CANNOT! 2 1 1 1 § Example: ____ HCl (aq) + ____ Zn (s) ____ H 2(g) + ____ Zn. Cl 2(aq) Reactants Elements H Cl Zn Products

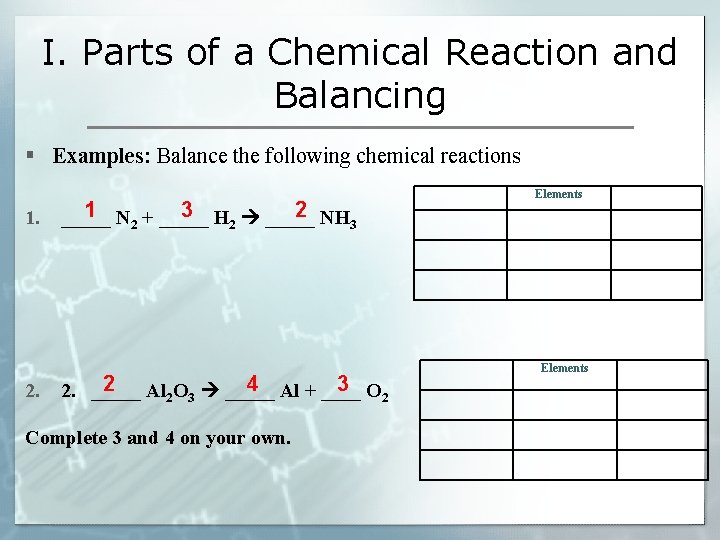
I. Parts of a Chemical Reaction and Balancing § Examples: Balance the following chemical reactions 1. 2. Elements 1 3 2 _____ N 2 + _____ H 2 _____ NH 3 2 4 3 2. _____ Al 2 O 3 _____ Al + ____ O 2 Complete 3 and 4 on your own. Elements

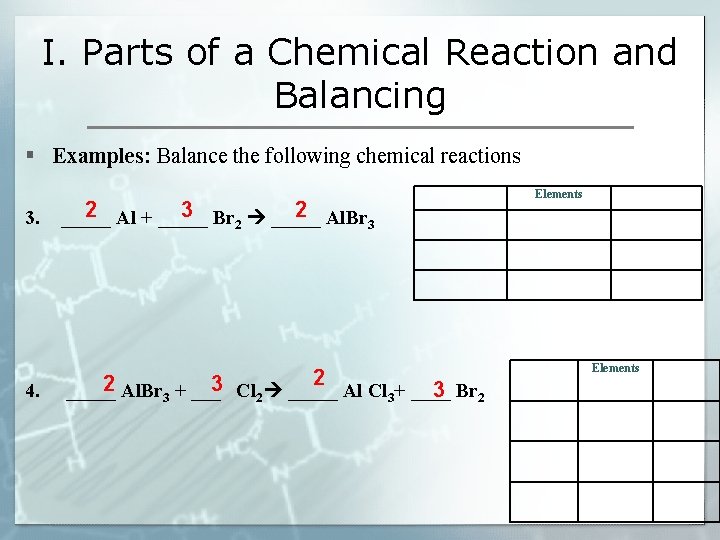
I. Parts of a Chemical Reaction and Balancing § Examples: Balance the following chemical reactions 3. 2 3 2 _____ Al + _____ Br 2 _____ Al. Br 3 2 2 3 4. _____ Al. Br 3 2 3 + ___ Cl 2 _____ Al Cl 3+ ____ Br Elements

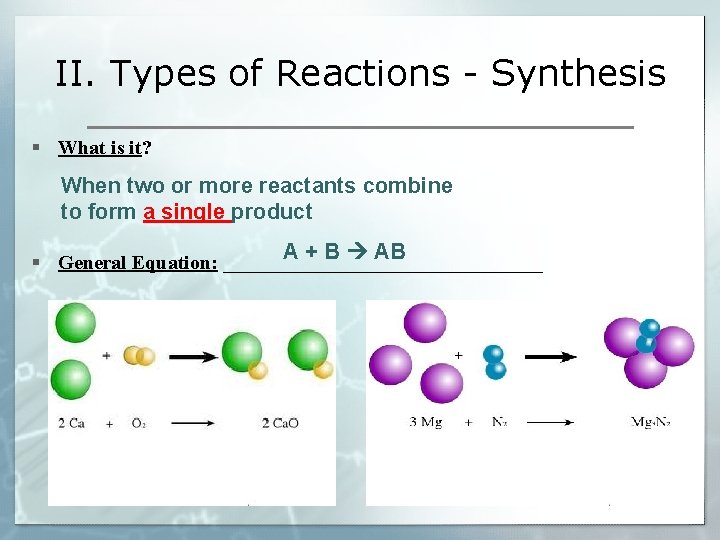
II. Types of Reactions - Synthesis § What is it? When two or more reactants combine to form a single product A + B AB § General Equation: ________________

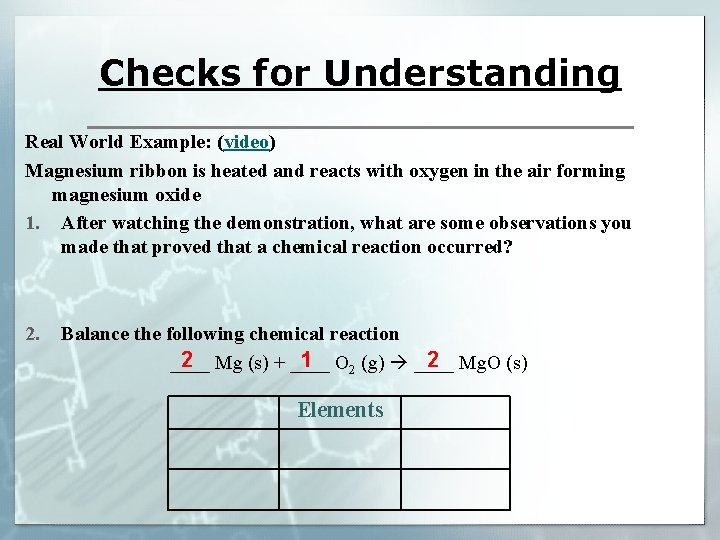
Checks for Understanding Real World Example: (video) Magnesium ribbon is heated and reacts with oxygen in the air forming magnesium oxide 1. After watching the demonstration, what are some observations you made that proved that a chemical reaction occurred? 2. Balance the following chemical reaction 2 1 2 ____ Mg (s) + ____ O 2 (g) ____ Mg. O (s) Elements

II. Types of Reactions - Synthesis C. Balancing: Balance the following synthesis reactions: 2 3 2 1. ____ S + ____ O 2 ____ SO 3 Elements 3 4 1 2. ____ C + ____ H 2 ____ C 3 H 8 Elements Complete 3 and 4 on your own and then check with your partner

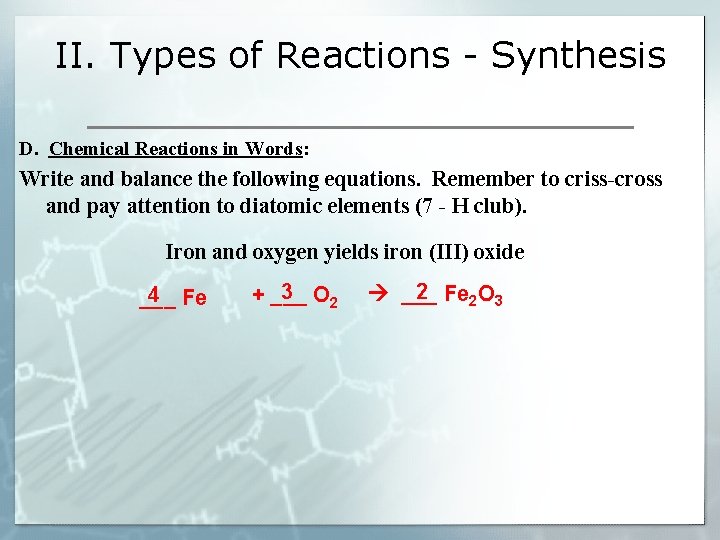
II. Types of Reactions - Synthesis D. Chemical Reactions in Words: Write and balance the following equations. Remember to criss-cross and pay attention to diatomic elements (7 - H club). Iron and oxygen yields iron (III) oxide 4 Fe ___ 3 O 2 + ___ 2 Fe 2 O 3 ___

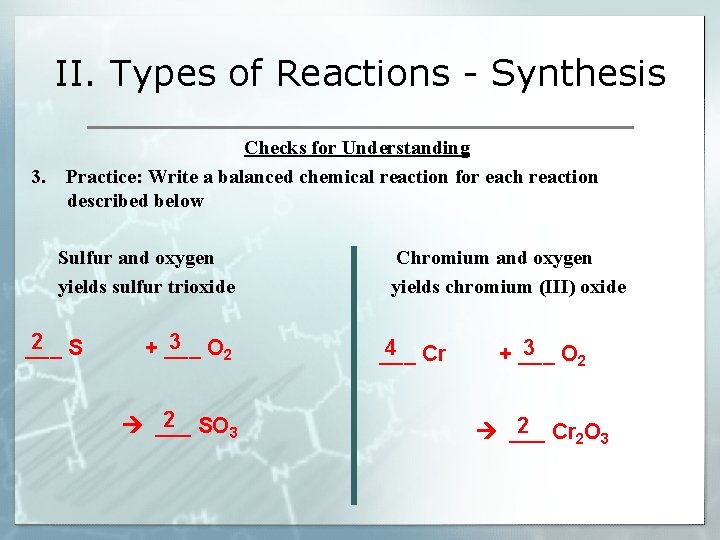
II. Types of Reactions - Synthesis Checks for Understanding 3. Practice: Write a balanced chemical reaction for each reaction described below Sulfur and oxygen yields sulfur trioxide Chromium and oxygen yields chromium (III) oxide 2 S ___ 3 O + ___ 2 2 SO ___ 3 4 Cr ___ 3 O + ___ 2 2 Cr O ___ 2 3

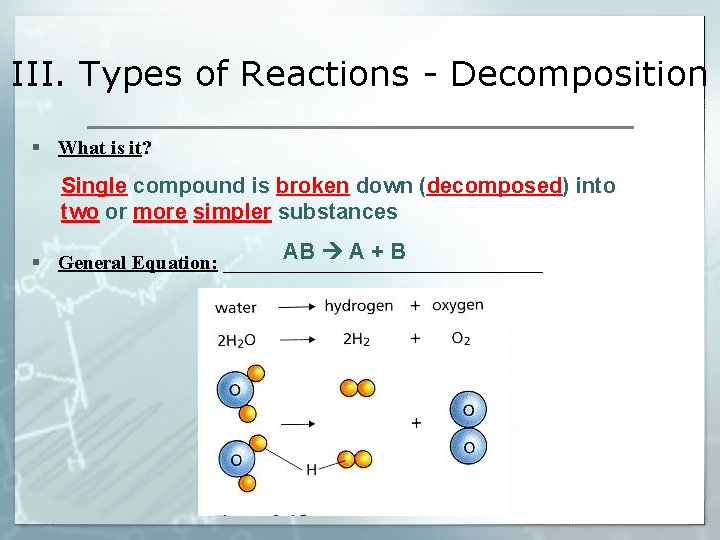
III. Types of Reactions - Decomposition § What is it? Single compound is broken down (decomposed) into two or more simpler substances AB A + B § General Equation: ________________

Checks for Understanding Real World Example: (video) Hydrogen peroxide decomposes into water and oxygen 1. After watching the demonstration, what are some observations you made that proved that a chemical reaction occurred? 2. Balance the following chemical reaction 2 2 1 ____ H 2 O 2 (l) ____ H 2 O (l) + ______ O 2 (g) Elements

III. Types of Reactions - Decomposition C. Balancing: Balance the following synthesis reactions: 2 2 1 1. ____ Hg. O ____ Hg + ____ O 2 2 2 1 2. ____ Ag. Cl ____ Ag + ____ Cl 2 Complete 3 and 4 on your own and then check with your partner Elements

III. Types of Reactions - Decomposition D. Chemical Reactions in Words: Write and balance the following equations. Remember to criss-cross and pay attention to diatomic elements (7 - H club). Nitrogen Triiodine decomposes to nitrogen and iodine ___ 2 NI 3 1 N ___ 2 3 I + ___ 2

Checks for Understanding 3. Practice: Write a balanced chemical reaction for each reaction described below Magnesium chloride decomposes into magnesium and chlorine ___ 1 Mg. Cl 2 1 Mg ___ Aluminum oxide decomposes into aluminum and oxygen ___ 2 Al 2 Cl 3 1 CI + ___ 2 4 Al ___ 3 CI + ___ 2

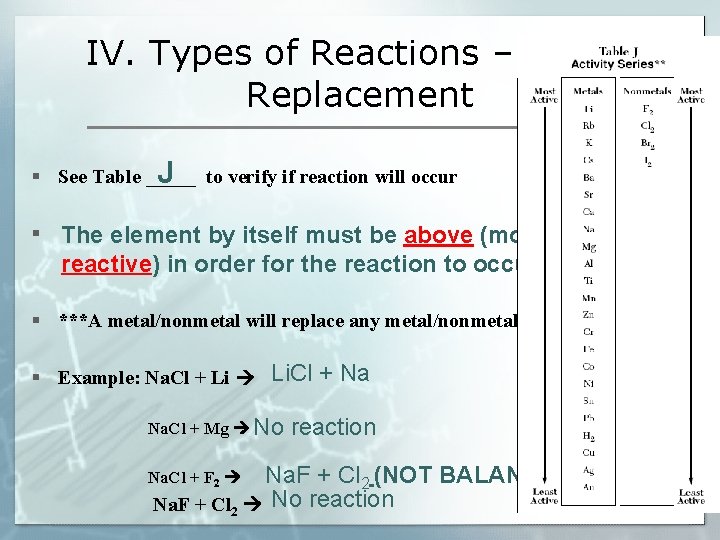
IV. Types of Reactions – Single Replacement A. What is it? Atoms of one element replace the atoms of a second element in a compound (metal switches with metal or nonmetal switches with nonmetal) § Always involves an element and a compound § Reaction will only occur if the single element is more reactive than the element in the compound (see Table J) B. General Equation: A + BX B + AX 2 K (s) + 2 H(OH) (l) H 2 (g) + 2 KOH (aq)

IV. Types of Reactions – Single Replacement § See Table _____ to verify if reaction will occur J § The element by itself must be above (more reactive) in order for the reaction to occur § ***A metal/nonmetal will replace any metal/nonmetal listed _____ below it § Example: Na. Cl + Li Li. Cl + Na Na. Cl + Mg No reaction Na. F + Cl 2 (NOT BALANCED) Na. F + Cl 2 No reaction Na. Cl + F 2

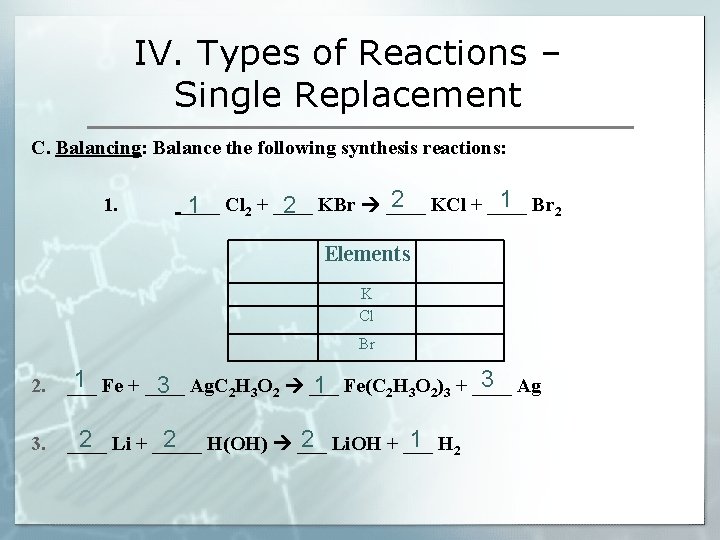
IV. Types of Reactions – Single Replacement C. Balancing: Balance the following synthesis reactions: 1. 2 1 ____ Cl 1 2 2 + ____ KBr ____ KCl + ____ Br 2 Elements K Cl Br 2. 1 3 ___ Fe + ____ Ag. C 3 1 2 H 3 O 2 ___ Fe(C 2 H 3 O 2)3 + ____ Ag 3. 2 2 2 1 2 ____ Li + _____ H(OH) ___ Li. OH + ___ H

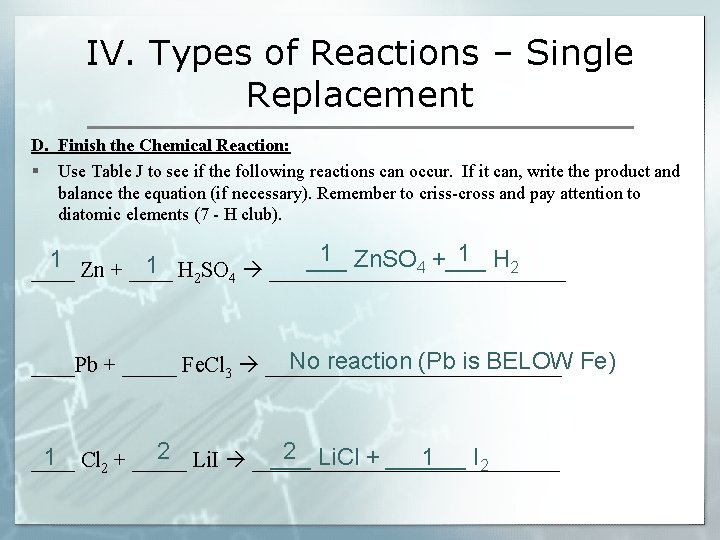
IV. Types of Reactions – Single Replacement D. Finish the Chemical Reaction: § Use Table J to see if the following reactions can occur. If it can, write the product and balance the equation (if necessary). Remember to criss-cross and pay attention to diatomic elements (7 - H club). 1 1 1 ___ Zn. SO +___ H 1 4 2 ____ Zn + ____ H 2 SO 4 ______________ No reaction (Pb is BELOW Fe) ____Pb + _____ Fe. Cl 3 ______________ 2 2 1 ___ Li. Cl + ______ I 1 ____ Cl + _____ Li. I ______________ 2 2

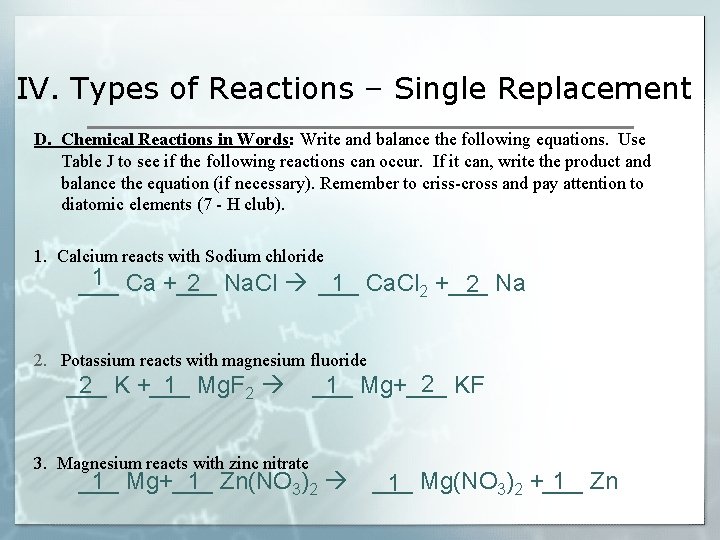
IV. Types of Reactions – Single Replacement D. Chemical Reactions in Words: Write and balance the following equations. Use Table J to see if the following reactions can occur. If it can, write the product and balance the equation (if necessary). Remember to criss-cross and pay attention to diatomic elements (7 - H club). 1. Calcium reacts with Sodium chloride 1 ___ Ca +___ Na. Cl 2 ___ Ca. Cl 1 2 2 +___ Na 2. Potassium reacts with magnesium fluoride ___ K +___ Mg. F 2 1 2 3. Magnesium reacts with zinc nitrate 2 ___ Mg+___ KF 1 1 ___ Mg+___ Zn(NO 1 3)2 ___ Mg(NO 1 1 3)2 +___ Zn

V. Types of Reactions – Double Replacement A. What is it? Involves an exchange of positive ions between two reacting ionic compounds § Occurs only if a solid, gas, or water is formed § Precipitates: A solid formed as a product of a reaction that does not dissolve in water (insoluble) § See Table ____: Examples – Cu. NO F (aq) 3 _____ Pb. Cl 2 ______ (s) Ca 3(PO 4)2 ____ (s) Ba(OH)2 ____ (aq)

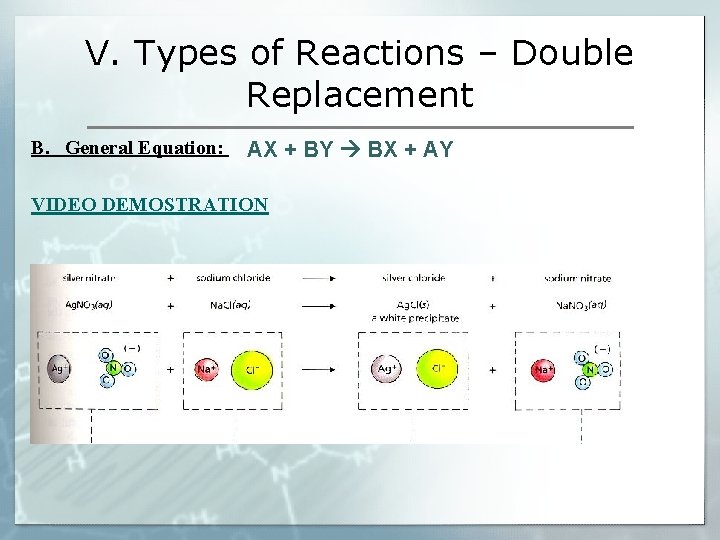
V. Types of Reactions – Double Replacement B. General Equation: AX + BY BX + AY VIDEO DEMOSTRATION

V. Types of Reactions – Double Replacement C. Balancing: Balance the following reactions 2 1 1 1 ____ Ba. Cl s aq 2 (aq) + ____ H 2 SO 4 (aq) ____ Ba. SO 4 ( ) + _____ HCl ( ) Elements Ba Cl H SO 4 1 3 aq ____ Al(NO s 3)3 (aq) + ___ Na. OH (aq) ____ Al(OH)3 ( )+ _____ Na. NO 3 ( ) 1 2 1 ____ Ca(OH) aq 1 aq l (s) 2 ( ) + ____ H 2 SO 4 ( ) ____ HOH ( ) + ____ Ca. SO 4

V. Types of Reactions – Double Replacement D. Finish the Chemical Reaction: Balance the following reactions § Write the products and balance the equation (if necessary). Remember to criss-cross. 2 1 1 1 ___ Na. NO (aq) +___ Cd. S (s) 3 ____ Na S (aq) + _____ Cd(NO ) (aq) ________________ 2 3 2 1 1 1 2 ___ Ba. SO ____Ba. Cl 2 (aq) + ____ H 2 SO 4 (aq) ____________________ 4 (s) +___ HCl (aq) 1 2 1 1 ___ Ba. CO 3 (s) +___ KCl (aq) ____ Ba. Cl 2 (aq) + _____ K 2 CO 3 (aq) _________________

V. Types of Reactions – Double Replacement § Chemical Reactions in Words: 1. Calcium Nitrate + Sodium Carbonate 1 1 ___ Ca(NO 3)2 (aq) +___ Na 2 CO 3 (aq) 2 ___ Ca. CO 1 3 (s) +___ Na. NO 3 (aq) 2. Aluminum nitrate and sodium hydroxide 1 3 ___ Al(NO 3)3 (aq) +___ Na. OH (aq) 3 ___ Na(NO 1 3) (aq)+___ Al(OH) 3 (S)

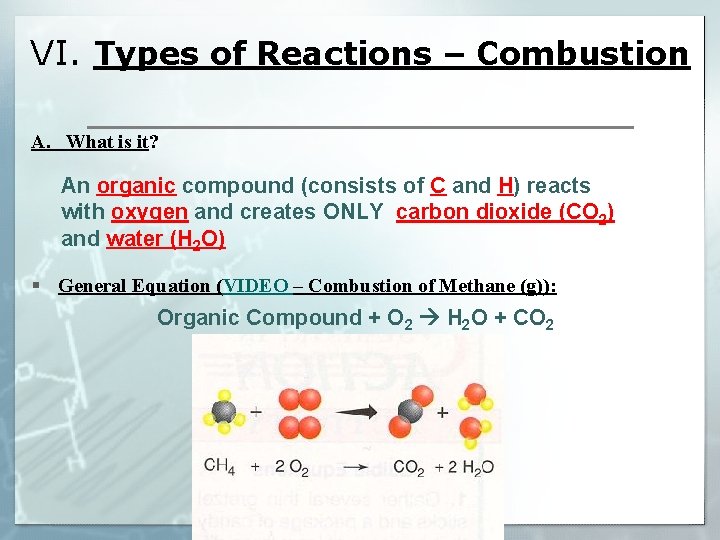
VI. Types of Reactions – Combustion A. What is it? An organic compound (consists of C and H) reacts with oxygen and creates ONLY carbon dioxide (CO 2) and water (H 2 O) § General Equation (VIDEO – Combustion of Methane (g)): Organic Compound + O 2 H 2 O + CO 2

VI. Types of Reactions – Combustion § Balancing: § Balance the following reactions: 1 7 6 4 § ____ C 4 H 12 + ____ O 2 ____ H 2 O + ______ CO 2 Elements C H O TRICKY!!! 2 7 2 ______ H § _____ C 6 4 2 H 6 + _____ O 2 O + _______ CO 2 2 3 4 2 § _____ CH 3 OH + ______ O 2 ______ H 2 O + ______CO 2

VIII. Stoichiometry § Applying math to chemical equations § Reasons: 1. To find the perfect “recipe” of reactants, producing the most products 2. To predict the mass or volume of every reactant and product § Coefficients: Tell you how many moles of the compound or element there are

VIII. Stoichiometry Think about this: The “recipe” for bread is: 3 cups of flour + 1 eggs 4 loafs of bread 1) How many loafs of bread is produced if you use 6 cups of flour? 8 loafs 2) How many eggs are needed to produce 12 loafs of bread? 3 eggs

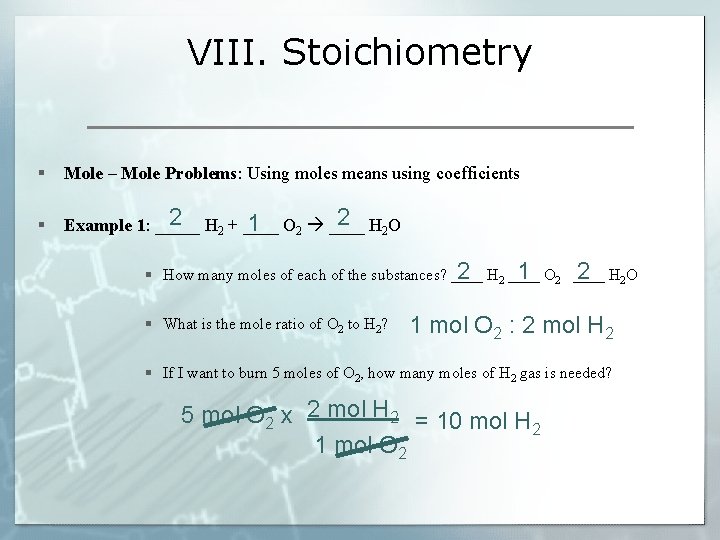
VIII. Stoichiometry § Mole – Mole Problems: Using moles means using coefficients § 2 2 + ____ O 2 2 O Example 1: _____ H 1 2 ____ H 2 2 ____ O 1 2 ____ H 2 2 O § How many moles of each of the substances? ____ H § What is the mole ratio of O 2 to H 2? 1 mol O 2 : 2 mol H 2 § If I want to burn 5 moles of O 2, how many moles of H 2 gas is needed? 5 mol O 2 x 2 mol H 2 = 10 mol H 2 1 mol O 2

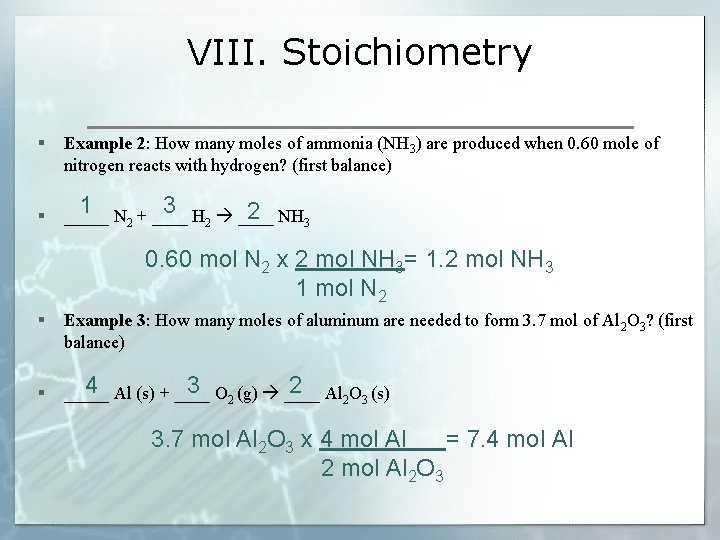
VIII. Stoichiometry § Example 2: How many moles of ammonia (NH 3) are produced when 0. 60 mole of nitrogen reacts with hydrogen? (first balance) § 2 _____ N 2 + ____ H 2 ____ NH 3 1 3 0. 60 mol N 2 x 2 mol NH 3= 1. 2 mol NH 3 1 mol N 2 § § Example 3: How many moles of aluminum are needed to form 3. 7 mol of Al 2 O 3? (first balance) 4 3 2 (g) ____ Al 2 _____ Al (s) + ____ O 2 O 3 (s) 3. 7 mol Al 2 O 3 x 4 mol Al = 7. 4 mol Al 2 mol Al O 2 3

Honors Chemistry § Mass-Mass Problems: When the mass of one substance in a reaction is given and you are asked to determine the mass of another substance in the reaction. The key to solving a mass -mass problem is having a correctly balanced equation. Example: How many grams of Na. Cl must be decomposed to yield 355 grams of Cl 2? 1. Write the balanced equation, given, and unknown. 2. Convert given to moles.

Honors Chemistry 3. Find moles of unknown using mole ratios from balanced equation. 4. Find mass of unknown. Same problem in 1 step using factor-label (dimensional analysis) method.

Honors Chemistry § Mass-Volume Problems: When the mass or volume of one substance in a reaction is given and you are asked to determine the mass or volume of another substance in the reaction. The key to solving a mass-volume problem is having a correctly balanced equation. Example: In the reaction, Zn + 2 HCl → Zn. Cl 2 + H 2, what volume of hydrogen is produced, at STP, when 3. 27 g of zinc are reacted with excess hydrochloric acid? 1. Write the balanced equation, given, and unknown. 2. Convert given to moles.

Honors Chemistry 3. Find moles of unknown using mole ratios from balanced equation. 4. Find volume of unknown using molar volume. Volume-Volume Problems: As you can imagine, these problems are very similar to mass problems. Example: In the reaction, 2 C 2 H 6 + 7 O 2 → 4 CO 2 + 6 H 2 O, how many liters of oxygen are required for the complete combustion of 15. 6 L of ethane (C 2 H 6)?

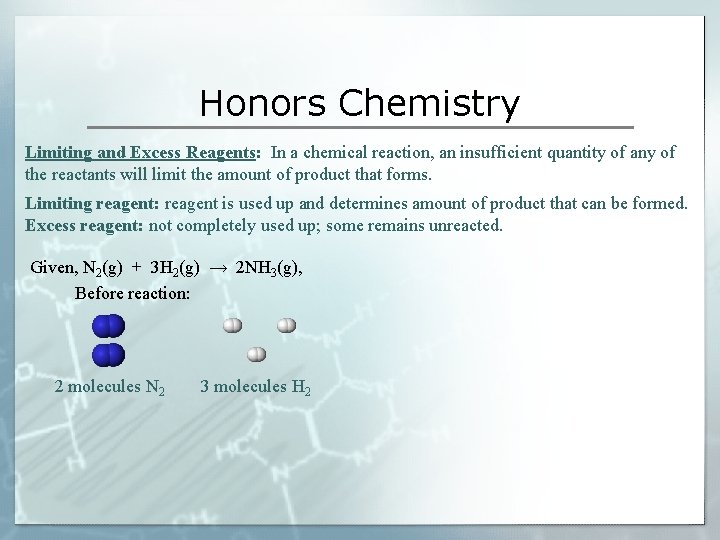
Honors Chemistry Limiting and Excess Reagents: In a chemical reaction, an insufficient quantity of any of the reactants will limit the amount of product that forms. Limiting reagent: reagent is used up and determines amount of product that can be formed. Excess reagent: not completely used up; some remains unreacted. Given, N 2(g) + 3 H 2(g) → 2 NH 3(g), Before reaction: 2 molecules N 2 3 molecules H 2

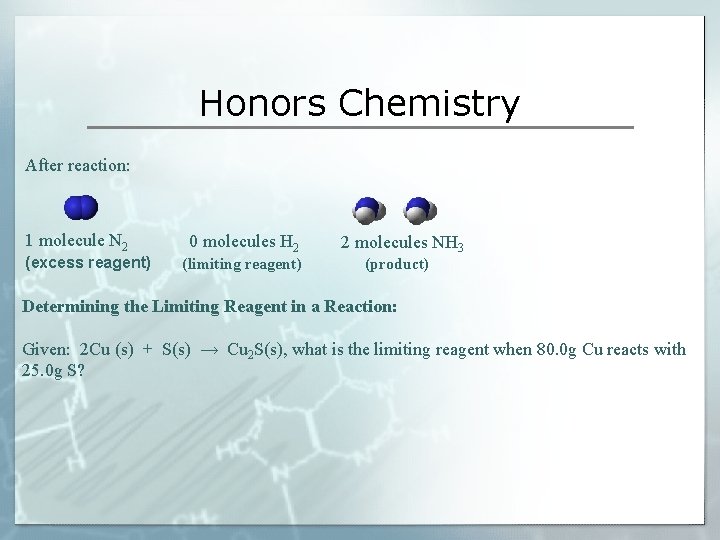
Honors Chemistry After reaction: 1 molecule N 2 (excess reagent) 0 molecules H 2 (limiting reagent) 2 molecules NH 3 (product) Determining the Limiting Reagent in a Reaction: Given: 2 Cu (s) + S(s) → Cu 2 S(s), what is the limiting reagent when 80. 0 g Cu reacts with 25. 0 g S?

Honors Chemistry Using a Limiting Reagent to Find the Quantity of a Product Given, 2 Cu (s) + S(s) → Cu 2 S(s), what is the maximum number of grams of Cu 2 S that can be formed when 80. 0 g Cu reacts with 25. 0 g S?