Unit 2 Chemical Reactions and Radioactivity 4 1







- Slides: 16

Unit 2: Chemical Reactions and Radioactivity 4. 1 Atomic Theory & Bonding

History of Atomic Theory § Around 500 BC, Greek philosophers originated the thought that all matter is composed of atoms. § The word atom comes from the Greek word atomos, which means “indivisible. ” § Around 1800: John Dalton revived discussion about the atom § 1898: J. J. Thomson discovered the electron § Soon after: Ernest Rutherford discovered the structure of the atom § Niels Bohr: proposed the concept of electron shells, created the “Bohr Model”

What is an Atom? § smallest particle of an element that still has the properties of that element § An atom = proton(s) + electron(s) + neutron(s) (PEN) § Atoms have a neutral charge § Fun Fact: 50 million atoms, lined up end-to-end = 1 cm

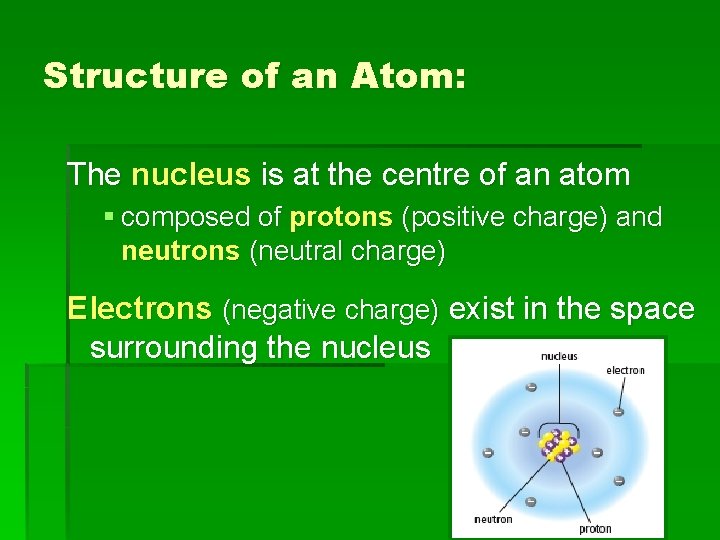
Structure of an Atom: The nucleus is at the centre of an atom § composed of protons (positive charge) and neutrons (neutral charge) Electrons (negative charge) exist in the space surrounding the nucleus

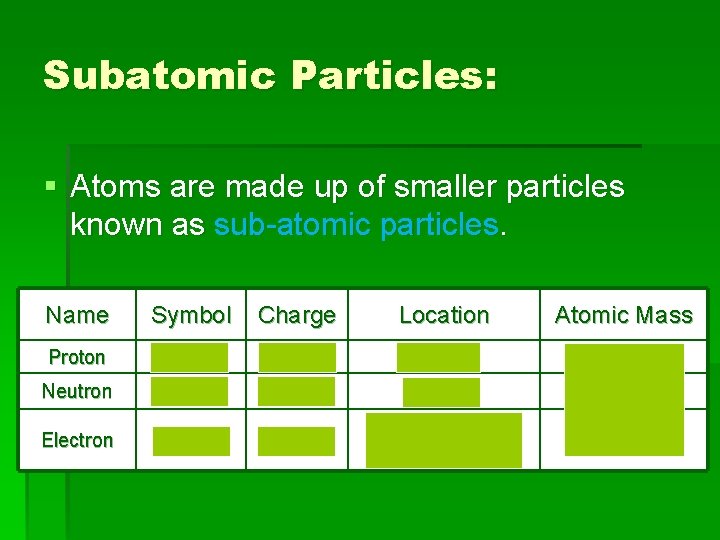
Subatomic Particles: § Atoms are made up of smaller particles known as sub-atomic particles. Name Symbol Charge Location Atomic Mass Proton p 1+ nucleus 1 AMU Neutron n 0 nucleus 1 AMU Electron e 1– area surrounding the nucleus 1/1836 (0)

§ Draw an atom! § Label the nucleus, protons, neutrons, electrons, and indicate the charge on each of them.

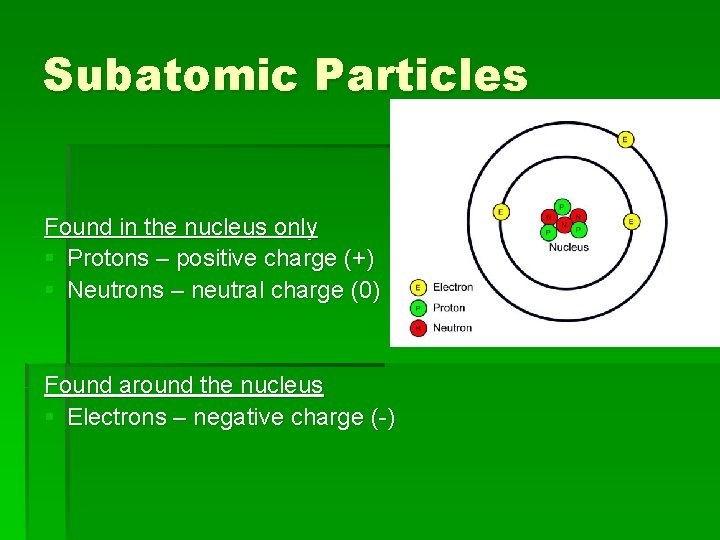
Subatomic Particles Found in the nucleus only § Protons – positive charge (+) § Neutrons – neutral charge (0) Found around the nucleus § Electrons – negative charge (-)

An ELEMENT is made up of one type of atom § The element, oxygen, is O § IONS are atoms with a charge. § The ion of oxygen is O 2 - Atom vs Ion Drawing on Board

§ Atoms can join together to form MOLECULES. The oxygen molecules are O 2 (groups of atoms) § COMPOUNDS are made up of several (2 or more) elements § Hydrogen and oxygen are elements § H 2 O is a compound

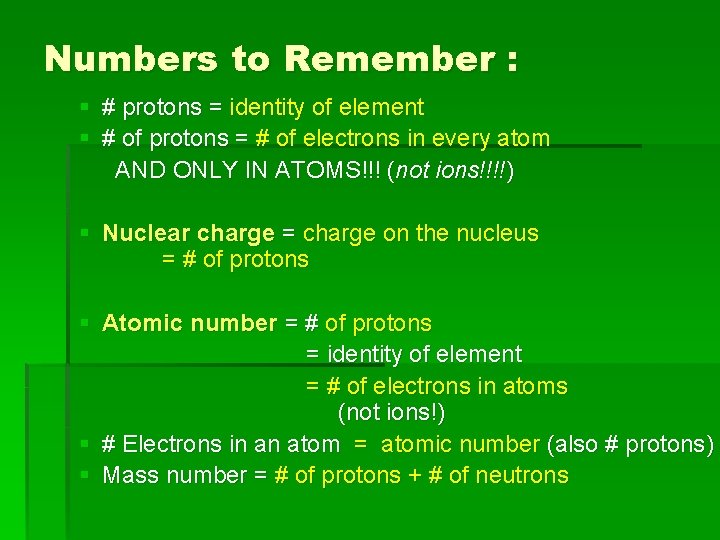
Numbers to Remember : § # protons = identity of element § # of protons = # of electrons in every atom AND ONLY IN ATOMS!!! (not ions!!!!) § Nuclear charge = charge on the nucleus = # of protons § Atomic number = # of protons = identity of element = # of electrons in atoms (not ions!) § # Electrons in an atom = atomic number (also # protons) § Mass number = # of protons + # of neutrons

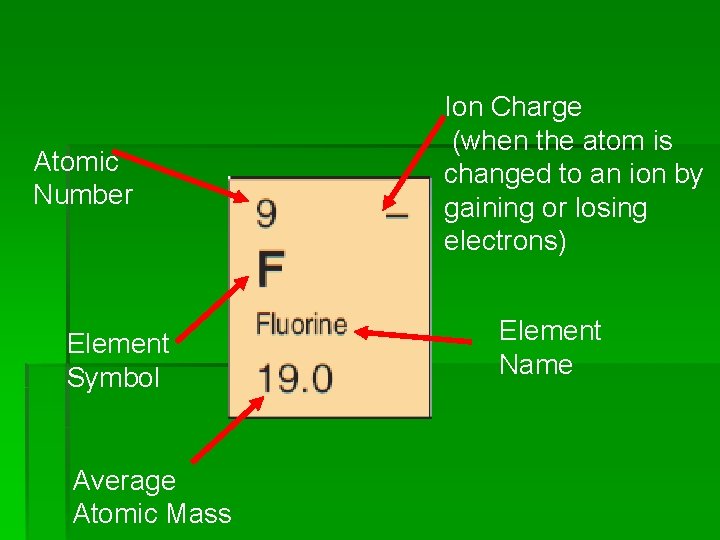
Atomic Number Element Symbol Average Atomic Mass Ion Charge (when the atom is changed to an ion by gaining or losing electrons) Element Name

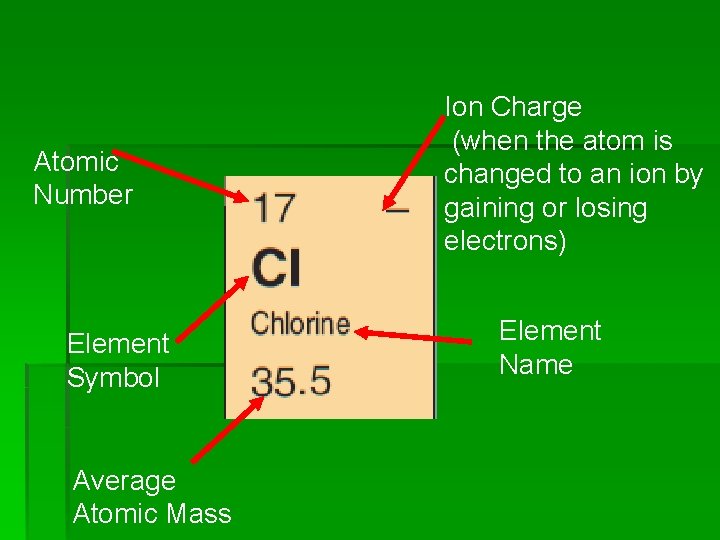
Atomic Number Element Symbol Average Atomic Mass Ion Charge (when the atom is changed to an ion by gaining or losing electrons) Element Name

Periodic Table Writing Activity

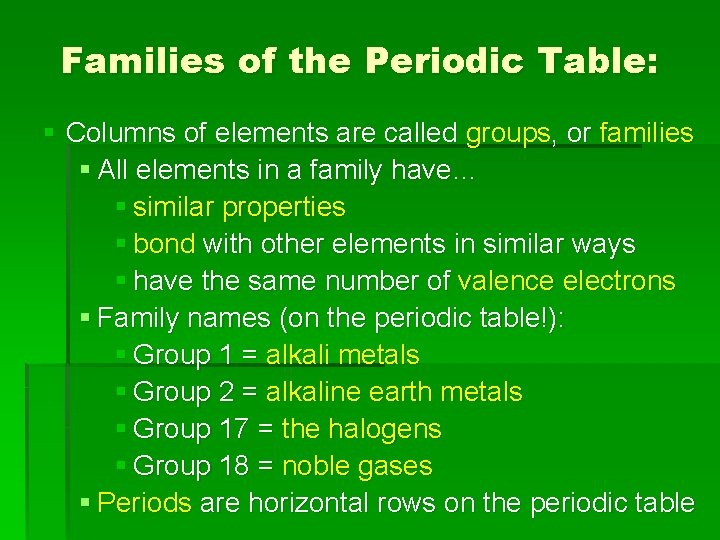
Families of the Periodic Table: § Columns of elements are called groups, or families § All elements in a family have… § similar properties § bond with other elements in similar ways § have the same number of valence electrons § Family names (on the periodic table!): § Group 1 = alkali metals § Group 2 = alkaline earth metals § Group 17 = the halogens § Group 18 = noble gases § Periods are horizontal rows on the periodic table

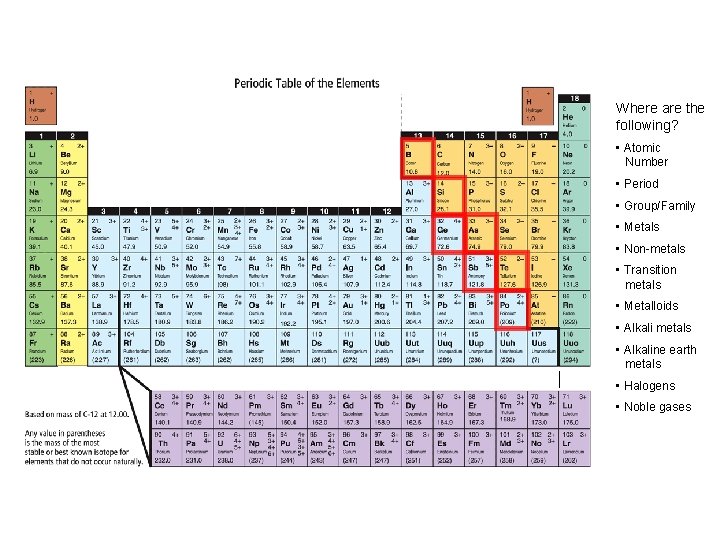
INCREASING REACTIVITY Where are the following? • Atomic Number • Period • Group/Family • Metals • Non-metals • Transition metals • Metalloids • Alkali metals • Alkaline earth metals • Halogens • Noble gases

Periodic Table & Ion Formation: § Atoms gain and lose electrons to form ions to become more stable (full valence shell) § Metals lose electrons & become positive ions (cations) § Some metals can have more than one charge (multivalent) § ie. Iron, Fe, loses either 2 (Fe 2+) or 3 (Fe 3+) electrons § Non-metals gain electrons & become negative ions (anions) § Atoms do this in an attempt to have the same number of valence electrons as the nearest noble gas – to become stable