UNIT 2 Chapter 4 Chemical Bonding and Properties
UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 Quantum Mechanics and Bonding Learning Goal: I will be able to show the shapes of s, p and d orbitals, and be able to show what is occuring in terms of the molecular orbital theory when bonding occurs. I will understand the difference between pi and sigma bonds, be able to locate them in a molecule, and be able to determine what type of hybridization occurs based on Lewis structures of molecules.
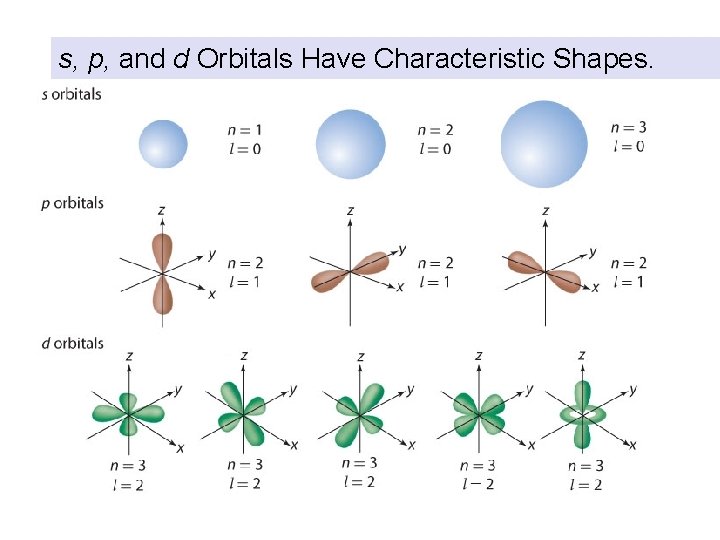
s, p, and d Orbitals Have Characteristic Shapes.
UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 Quantum Mechanics and Bonding Quantum mechanics is used to explain and describe chemical bonding. It is also used to account for shapes of molecules. Valence Bond (VB) Theory explains bond formation and molecular shapes based on orbital overlap. • The region of overlap has a maximum capacity of two electrons, which have opposite spins. • There should be maximum overlap of orbitals, since the greater the overlap, the stronger and more stable the bond. • Atomic orbital hybridization is used to help explain the shapes of some molecules.
UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 Quantum Mechanics and Bonding Molecular Orbital (MO) Theory explains bond formation and molecular shapes based on the formation of new molecular orbitals. According to MO theory: • Covalent bond formation involves atomic orbital overlap that results in formation of new orbitals called molecular orbitals. • Molecular orbitals have shapes and energy levels that are different from those of atomic orbitals. • The electrons in molecular orbitals are delocalized throughout the orbital.
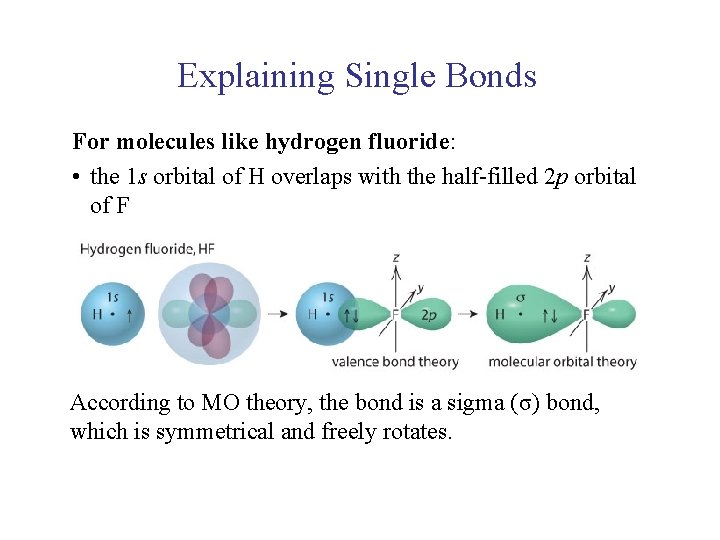
UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 Explaining Single Bonds For molecules like hydrogen fluoride: • the 1 s orbital of H overlaps with the half-filled 2 p orbital of F According to MO theory, the bond is a sigma (σ) bond, which is symmetrical and freely rotates.
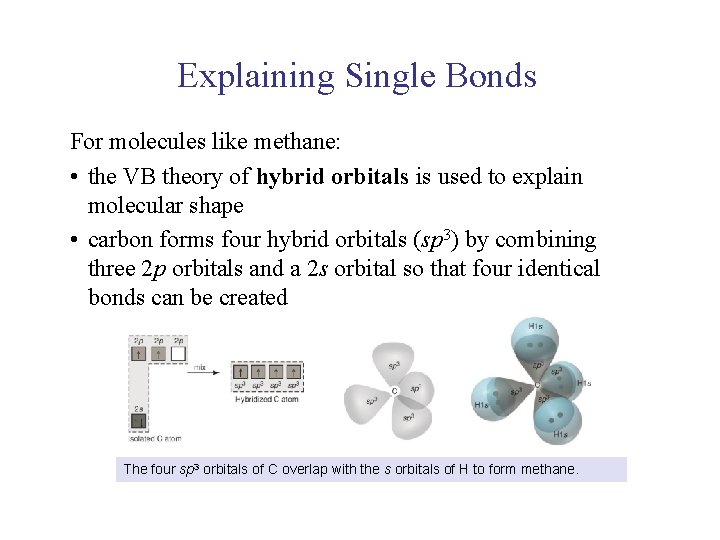
UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 Explaining Single Bonds For molecules like methane: • the VB theory of hybrid orbitals is used to explain molecular shape • carbon forms four hybrid orbitals (sp 3) by combining three 2 p orbitals and a 2 s orbital so that four identical bonds can be created The four sp 3 orbitals of C overlap with the s orbitals of H to form methane.
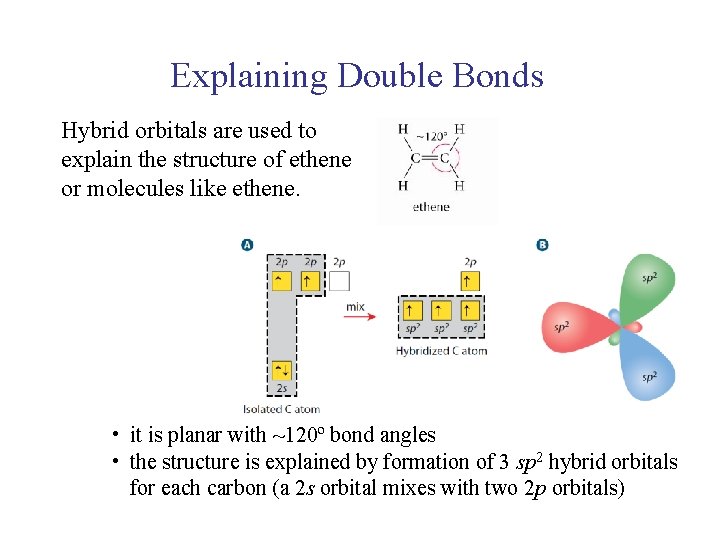
UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 Explaining Double Bonds Hybrid orbitals are used to explain the structure of ethene or molecules like ethene. • it is planar with ~120º bond angles • the structure is explained by formation of 3 sp 2 hybrid orbitals for each carbon (a 2 s orbital mixes with two 2 p orbitals)
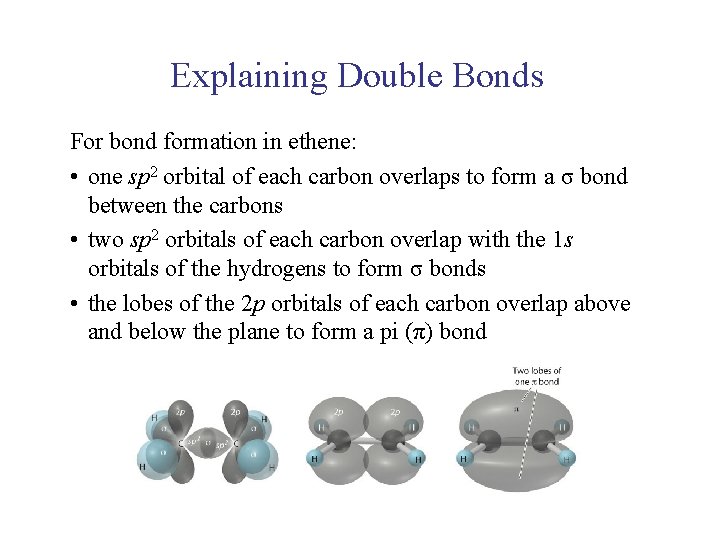
UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 Explaining Double Bonds For bond formation in ethene: • one sp 2 orbital of each carbon overlaps to form a σ bond between the carbons • two sp 2 orbitals of each carbon overlap with the 1 s orbitals of the hydrogens to form σ bonds • the lobes of the 2 p orbitals of each carbon overlap above and below the plane to form a pi (π) bond
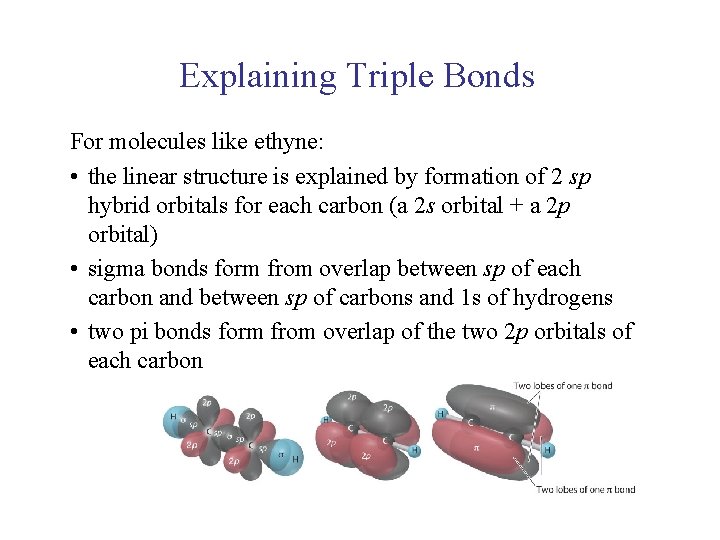
UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 Explaining Triple Bonds For molecules like ethyne: • the linear structure is explained by formation of 2 sp hybrid orbitals for each carbon (a 2 s orbital + a 2 p orbital) • sigma bonds form from overlap between sp of each carbon and between sp of carbons and 1 s of hydrogens • two pi bonds form from overlap of the two 2 p orbitals of each carbon
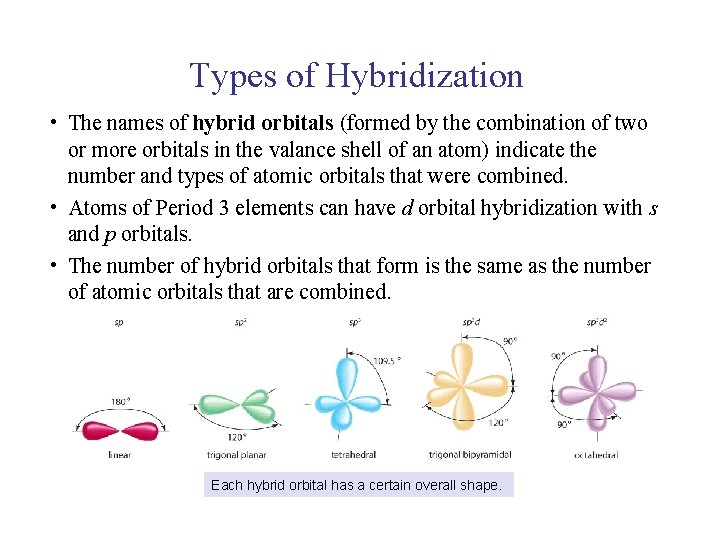
UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 Types of Hybridization • The names of hybrid orbitals (formed by the combination of two or more orbitals in the valance shell of an atom) indicate the number and types of atomic orbitals that were combined. • Atoms of Period 3 elements can have d orbital hybridization with s and p orbitals. • The number of hybrid orbitals that form is the same as the number of atomic orbitals that are combined. Each hybrid orbital has a certain overall shape.

UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 Example: For chloroethene: a) Draw the Lewis structure b) Locate all sigma and pi bonds c) Draw the orbital diagram for Carbon d) Draw the hybridization that must occur for carbon to bond with H and C e) Name what type of hybridization has occured

UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 You Try For propene: a) Draw the Lewis structure b) Locate all sigma and pi bonds c) Draw the orbital diagram for Carbon d) Draw the hybridization that must occur for carbon to bond with H and C e) Name what type of hybridization has occured
UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 Power. Point Summary
UNIT 2 Chapter 4: Chemical Bonding and Properties of Matter Section 4. 1 How Did We Do? Learning Goal: I will be able to show the shapes of s, p and d orbitals, and be able to show what is occuring in terms of the molecular orbital theory when bonding occurs. I will understand the difference between pi and sigma bonds, be able to locate them in a molecule, and be able to determine what type of hybridization occurs based on Lewis structures of molecules.
- Slides: 14