Unit 2 Biochemistry 2 2 Chemical Bonding Chemical
Unit 2: Biochemistry 2. 2 Chemical Bonding
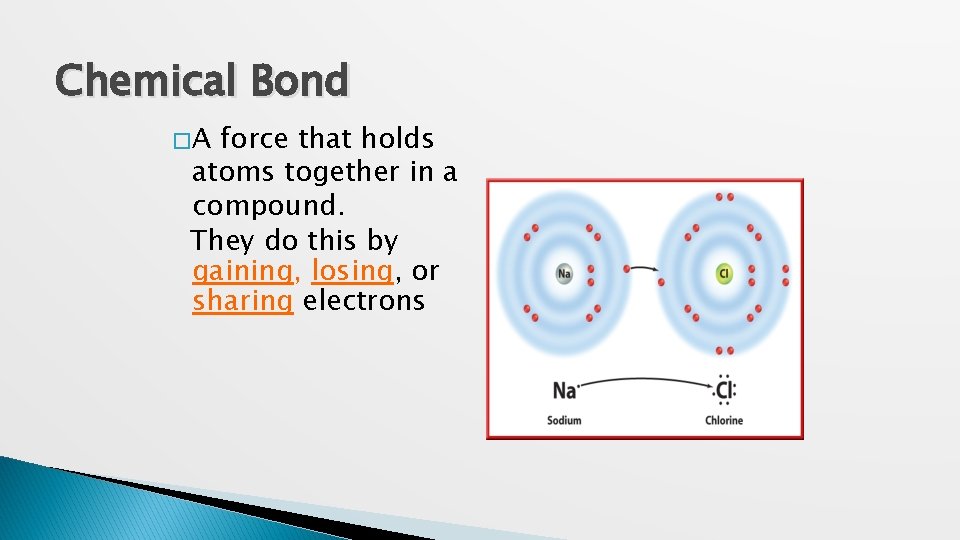
Chemical Bond �A force that holds atoms together in a compound. They do this by gaining, losing, or sharing electrons
Covalent bond � Covalent bond: the attraction that forms between atoms when they share electrons. ◦ Forms a neutral compound called a molecule ◦ Usually occurs between nonmetals ◦ Single covalent bonds – sharing of 2 electrons (one from each atom in the bond) ◦ Multiple bonds – sharing of more than one electron by each atom

Single Bonds

Multiple Bonds

Ionic Bond � Ionic Bond: the force of attraction between the opposite charges of ions, electrons are transferred. ◦ Occurs when electrons are lost or gained (transferred between atoms) ◦ Usually formed between metals and nonmetals �Ex. Salt Na. Cl
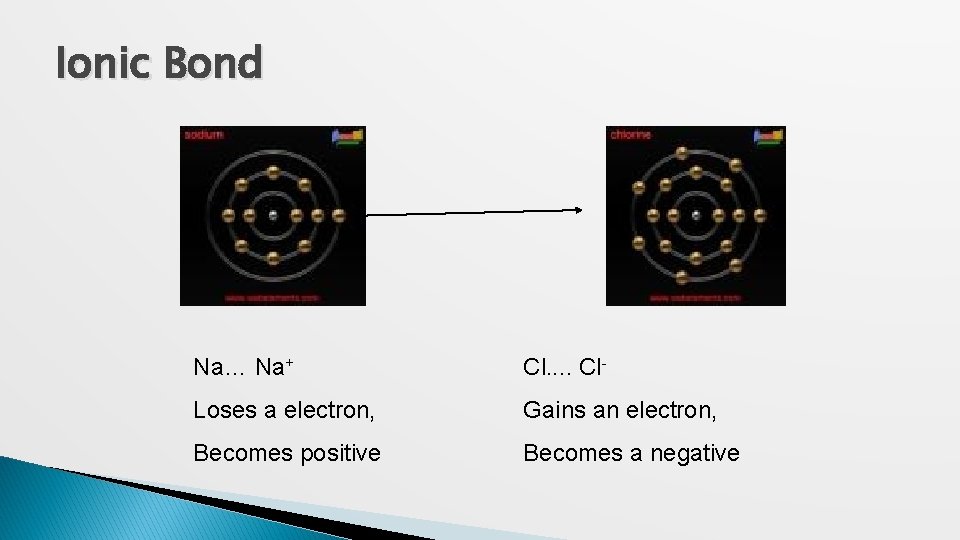
Ionic Bond Na… Na+ Cl. . Cl- Loses a electron, Gains an electron, Becomes positive Becomes a negative


Hydrogen Bond � Hydrogen Bond: a hydrogen atom bonded to one molecule is attracted to the slightly negative area (often N or O) of another molecule. ◦ Very weak individual bond. ◦ Can be a “strong” force if there are many H bonds.
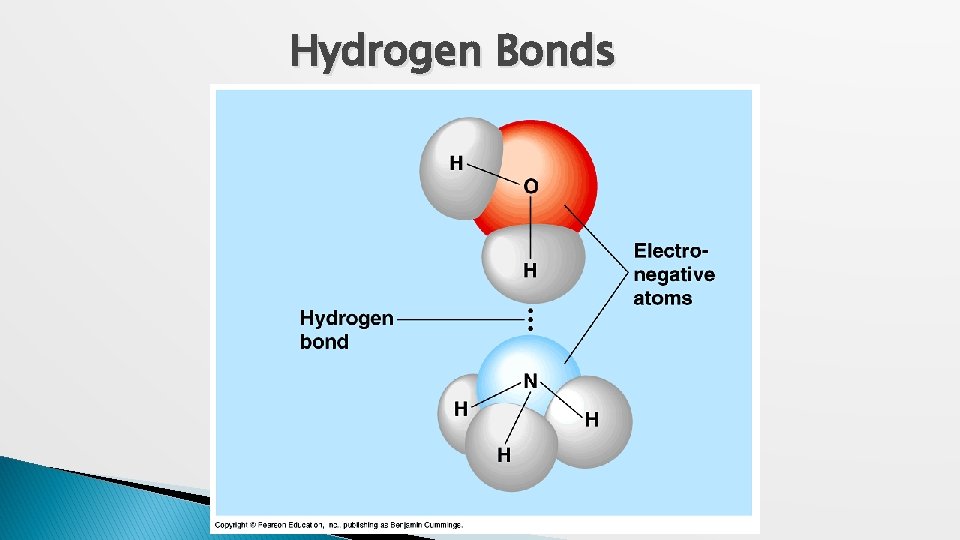
Hydrogen Bonds
Polar/Nonpolar Molecules �Polar Covalent Molecules: molecule with a slightly positive and a slightly negative end although the overall molecule is neutral ◦ Ex. H 2 O �Nonpolar Molecules: a molecule in which the electrons are shared evenly (does not have oppositely charged ends) � 2 molecules of the same atom or symmetric molecules ◦ Ex. CCl 4 ◦ N 2
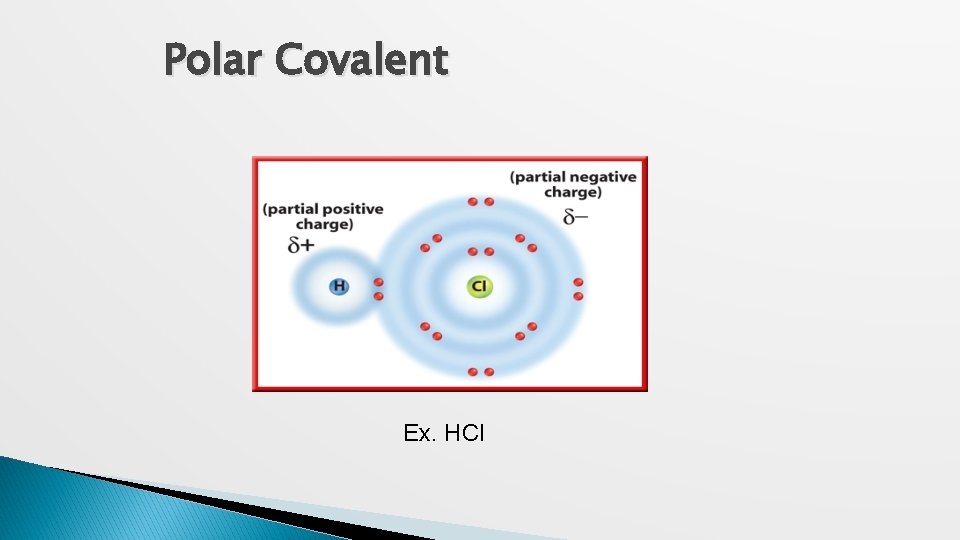
Polar Covalent Ex. HCl
Bonding Analogy � Create a picture analogy to represent the three different kinds of bonds. An analogy is a comparison of two things. � Key Ideas: ◦ Covalent: atoms share electrons. ◦ Ionic: atoms transfer electrons from one atom to another. ◦ Hydrogen: opposite poles (positive end and negative end) of molecules attract.
- Slides: 13