Unit 2 Atoms Scientists to Know CHADWICK THOMSON











![Classical Era John Dalton [really famous] (1766 -1844) o Dalton returns to Democritus’ ideas Classical Era John Dalton [really famous] (1766 -1844) o Dalton returns to Democritus’ ideas](https://slidetodoc.com/presentation_image_h2/ea84301100d07d7660888233e255d98f/image-14.jpg)






- Slides: 29

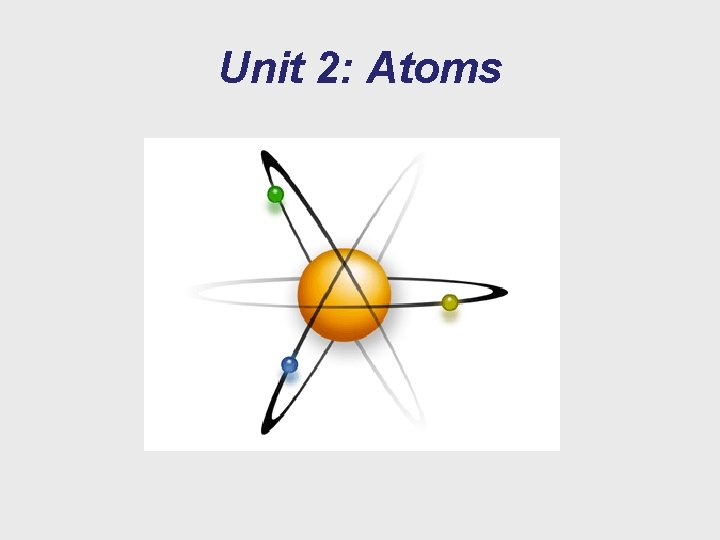
Unit 2: Atoms

Scientists to Know … CHADWICK THOMSON RUTHERFORD DEMOCRITUS BOHR HEISENBERG DALTON

The History of Discovering the Atom The Timeline of Discovery 1. 2. 3. 4. 5. Philosophical Era Alchemical Era Classical Era Subatomic Era Modern Era – For later study… The Intermediate Atomic Models 1. Uncuttable Model 2. --3. The Dalton Sphere Model 4. The Plumb Pudding Model The Planetary Model 5. The Quantum Model

Brainstorm about this era? THE PHILOSOPHICAL ERA (CIRCA 500~300 BCE)

A time when logic ruled the land… THE PHILOSOPHICAL ERA (CIRCA 500~300 BCE)

Philosophical Era (Ancient Greece) o Their ideas were based on logic, without experimental support (as was common in that time) o Age of Thinking

Philosophical Era Democritus (460 -370 BCE) o Argued that matter was made of small, indivisible particles o Called the small particles “atomos” meaning “that which cannot be divided” o Believed properties of matter came from the properties of the “atomos”

Brainstorm about this era? ALCHEMICAL ERA (300 BCE ~ 1400 CE)

The “Dark Ages” of Chemistry where early chemists had to work in secret and encode their findings for fear of persecution ALCHEMICAL ERA (300 BCE ~ 1400 CE)

Alchemical Era Alchemy o the closest thing to the study of chemistry for nearly two thousand years o Very mystical study and experimentation with the elements and what was perceived as magic o Study was illegal, findings hidden in code

Alchemical Era Elements in Alchemy o Alchemists studied many different materials, and their properties, in order to find a way to turn lead into gold and achieve immortality

Brainstorm about this era? THE CLASSICAL ERA (1400 CE – 1887 CE)

The printing press brings the widespread transfer and acquisition of knowledge THE CLASSICAL ERA (1400 CE – 1887 CE)
![Classical Era John Dalton really famous 1766 1844 o Dalton returns to Democritus ideas Classical Era John Dalton [really famous] (1766 -1844) o Dalton returns to Democritus’ ideas](https://slidetodoc.com/presentation_image_h2/ea84301100d07d7660888233e255d98f/image-14.jpg)
Classical Era John Dalton [really famous] (1766 -1844) o Dalton returns to Democritus’ ideas in 1803 with four arguments I. All matter is made up of tiny particles called atoms II. All atoms of a given element are identical to one another and different from atoms of other elements III. Atoms of two or more different elements combine to form compounds. IV. A chemical reaction involves the rearrangement, separation, or combination of atoms. Atoms are never created nor destroyed during a chemical reaction. The Atom John Dalton

Brainstorm about this era? THE SUBATOMIC ERA (1897 CE – 1932 CE)

The relatively quick discovery of things smaller than the once “indivisible” atom - EXPERIMENTAION THE SUBATOMIC ERA (1897 CE – 1932 CE)

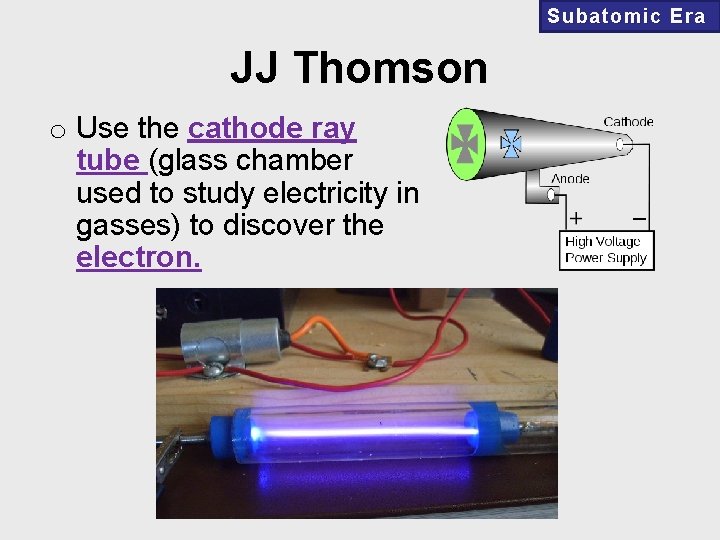
Subatomic Era JJ Thomson o Use the cathode ray tube (glass chamber used to study electricity in gasses) to discover the electron.

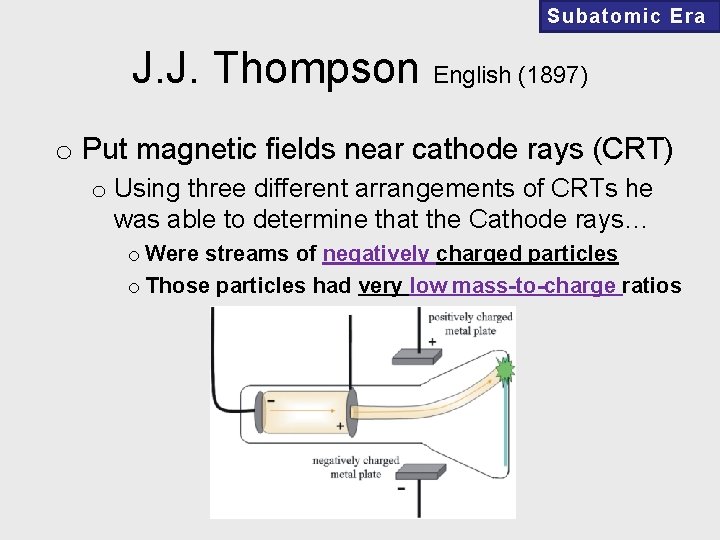
Subatomic Era J. J. Thompson English (1897) o Put magnetic fields near cathode rays (CRT) o Using three different arrangements of CRTs he was able to determine that the Cathode rays… o Were streams of negatively charged particles o Those particles had very low mass-to-charge ratios

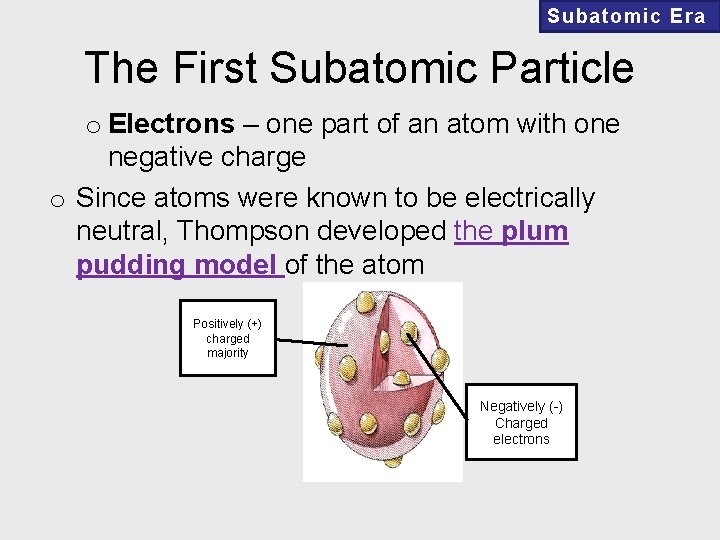
Subatomic Era The First Subatomic Particle o Electrons – one part of an atom with one negative charge o Since atoms were known to be electrically neutral, Thompson developed the plum pudding model of the atom Positively (+) charged majority Negatively (-) Charged electrons

Subatomic Era Ernest Rutherford New Zealander (1910) o Rutherford worked with radiation and had heard of Thompson’s plumb pudding model o He wanted to use radiation to prove Thompson’s model o With the help from Marie Curie, he shot alpha particles (+) at an ultra-thin piece of gold foil, with a Geiger counter on the other side Ernest Rutherford New Zealand Marie Curie Polish/ French

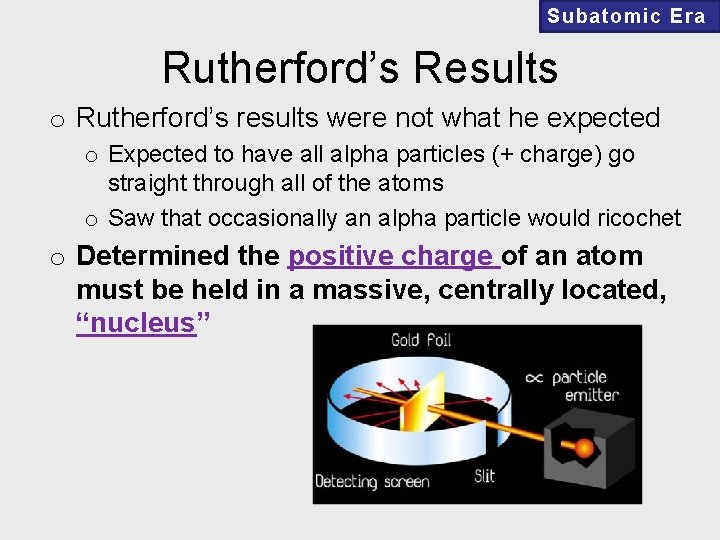
Subatomic Era Rutherford’s Results o Rutherford’s results were not what he expected o Expected to have all alpha particles (+ charge) go straight through all of the atoms o Saw that occasionally an alpha particle would ricochet o Determined the positive charge of an atom must be held in a massive, centrally located, “nucleus”

Subatomic Era The Second Subatomic Particle o After more experiments the second subatomic particle was formally named (1911) o Proton: The massive subatomic particle, within the nucleus of an atom, with a single positive charge

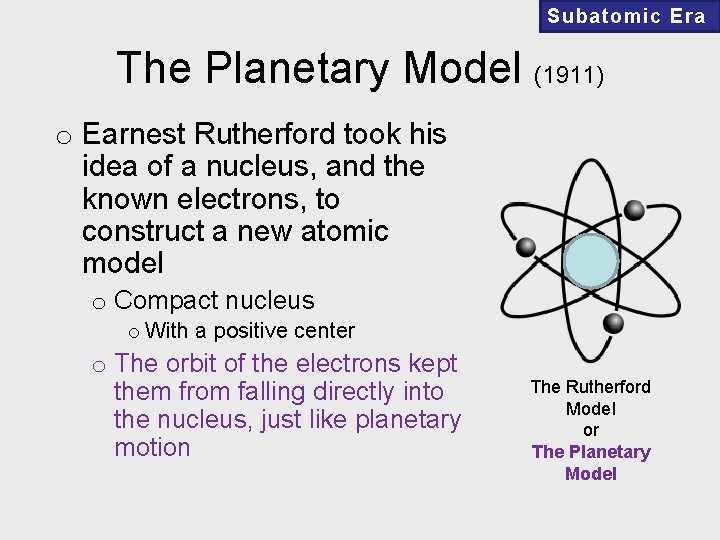
Subatomic Era The Planetary Model (1911) o Earnest Rutherford took his idea of a nucleus, and the known electrons, to construct a new atomic model o Compact nucleus o With a positive center o The orbit of the electrons kept them from falling directly into the nucleus, just like planetary motion The Rutherford Model or The Planetary Model

Subatomic Era The Third Subatomic Particle o Missing mass in the nucleus o James Chadwick – determined that a another subatomic particle must be in the nucleus with the protons o Called this subatomic particle: neutron because it has NO charge. James Chadwick English

Brainstorm about this era? THE MODERN ERA (1900 CE – PRESENT)

The Quark Era starts in 1964, but that advance can be regarded as outside the realm of chemistry – instead a part of nuclear physics THE MODERN ERA (1900 CE – PRESENT)

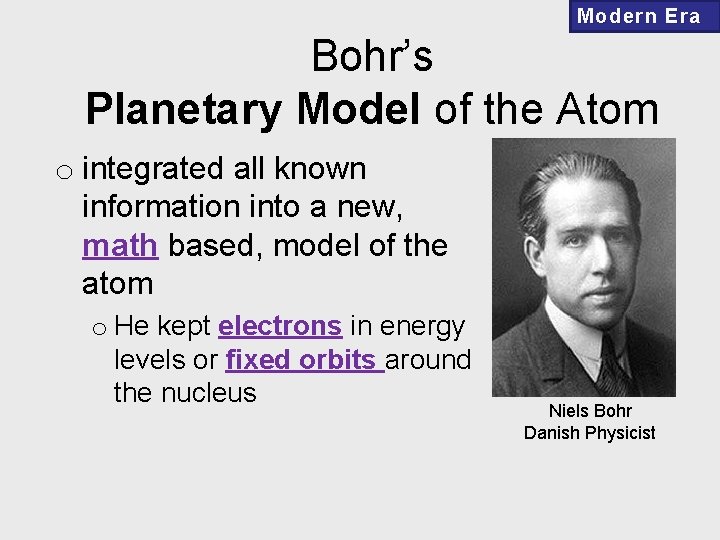
Modern Era Bohr’s Planetary Model of the Atom o integrated all known information into a new, math based, model of the atom o He kept electrons in energy levels or fixed orbits around the nucleus Niels Bohr Danish Physicist

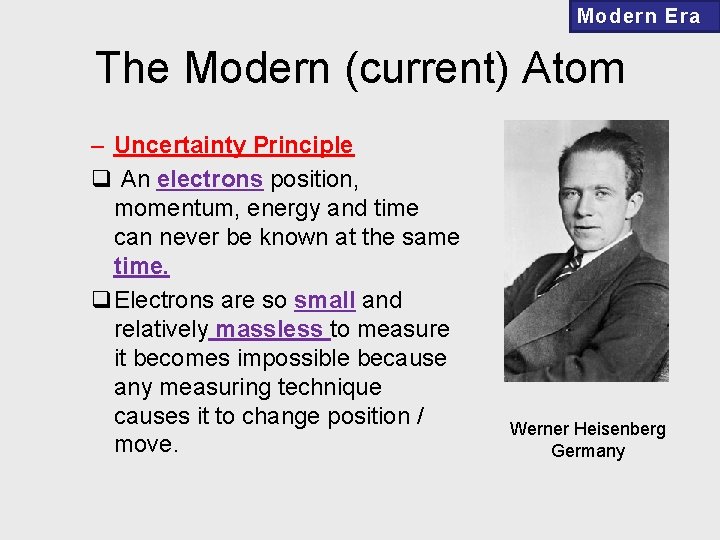
Modern Era The Modern (current) Atom – Uncertainty Principle q An electrons position, momentum, energy and time can never be known at the same time. q. Electrons are so small and relatively massless to measure it becomes impossible because any measuring technique causes it to change position / move. Werner Heisenberg Germany

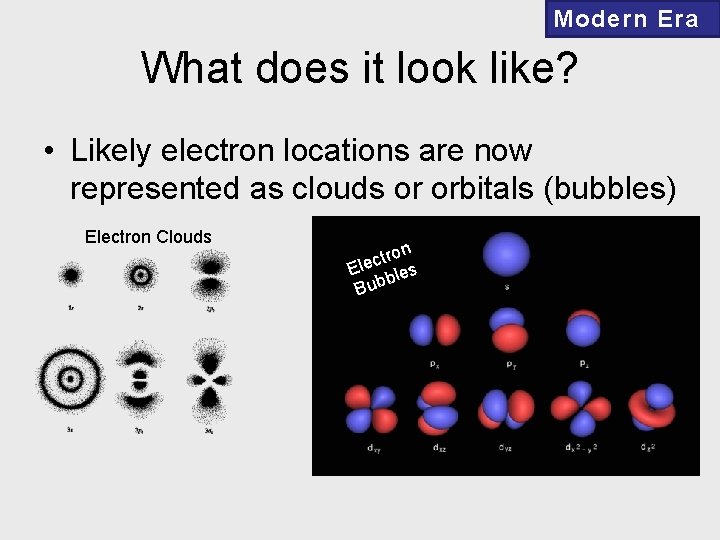
Modern Era What does it look like? • Likely electron locations are now represented as clouds or orbitals (bubbles) Electron Clouds tron c e l E bles b u B