Unit 2 Atomic Theory Atomic Symbols Naming Compounds

Unit 2: Atomic Theory, Atomic Symbols, Naming Compounds Chapter 3, 5, & 6

Key Concepts • • • Percent Yield, Percent Error Theory, Law of Conservation of Energy, Law of Conservation of Mass Lab Atomic Theory: Dalton, Thompson, Rutherford, Bohr, Schrodinger Models Atomic Number, Atomic Mass Subatomic Particles: Protons, Neutrons, Electrons Isotopes, Ions Compounds: Ionic, Covalent, Metallic Metals, Nonmetals, Transition Metals Naming Covalent Compounds Naming Ionic Compounds
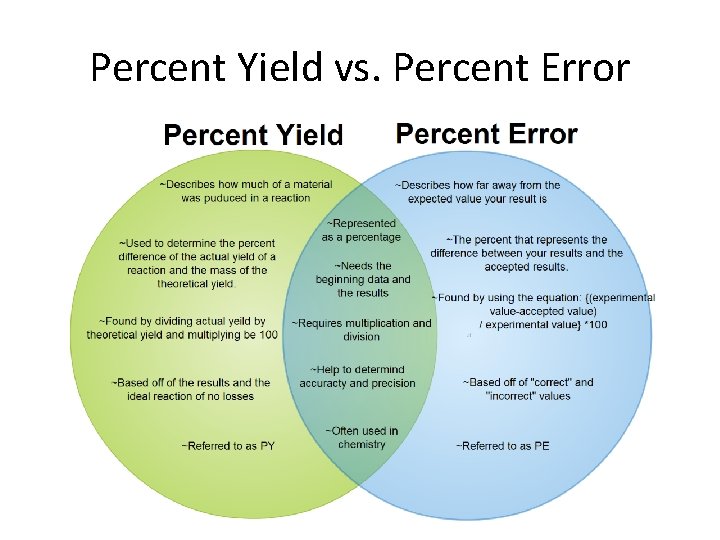
Percent Yield vs. Percent Error
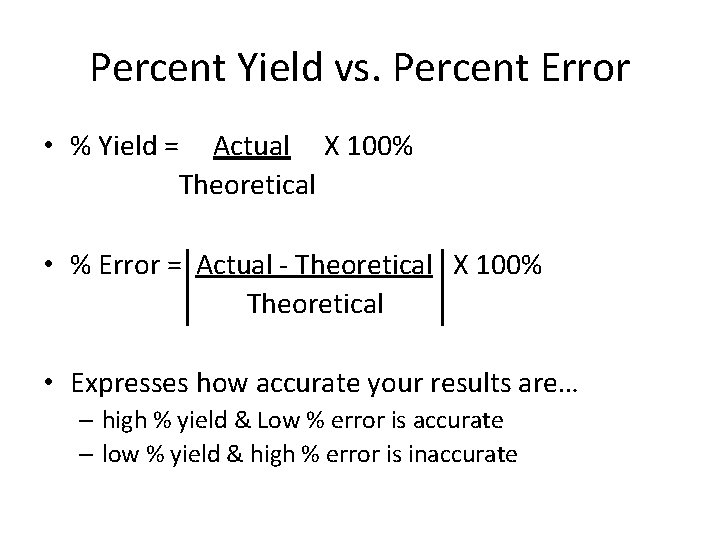
Percent Yield vs. Percent Error • % Yield = Actual X 100% Theoretical • % Error = Actual - Theoretical X 100% Theoretical • Expresses how accurate your results are… – high % yield & Low % error is accurate – low % yield & high % error is inaccurate
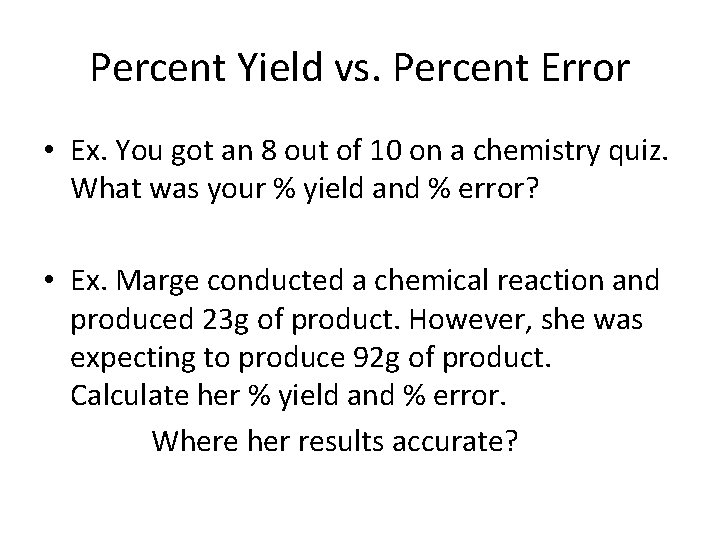
Percent Yield vs. Percent Error • Ex. You got an 8 out of 10 on a chemistry quiz. What was your % yield and % error? • Ex. Marge conducted a chemical reaction and produced 23 g of product. However, she was expecting to produce 92 g of product. Calculate her % yield and % error. Where her results accurate?
Theory vs. Law • Theory: explains the cause of events in the natural world – Darwin’s Theory of Evolution by Natural Selection explains how organisms change over time • Law: describes events in the natural world – Newton’s Law of Universal Gravitation describes gravity

Law of Conservation of Energy • Law of Conservation of Energy: energy is not created or destroyed, only transferred
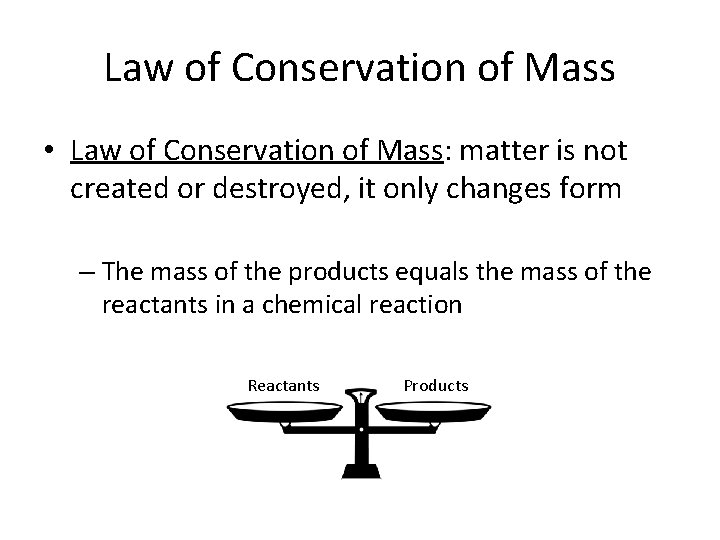
Law of Conservation of Mass • Law of Conservation of Mass: matter is not created or destroyed, it only changes form – The mass of the products equals the mass of the reactants in a chemical reaction Reactants Products
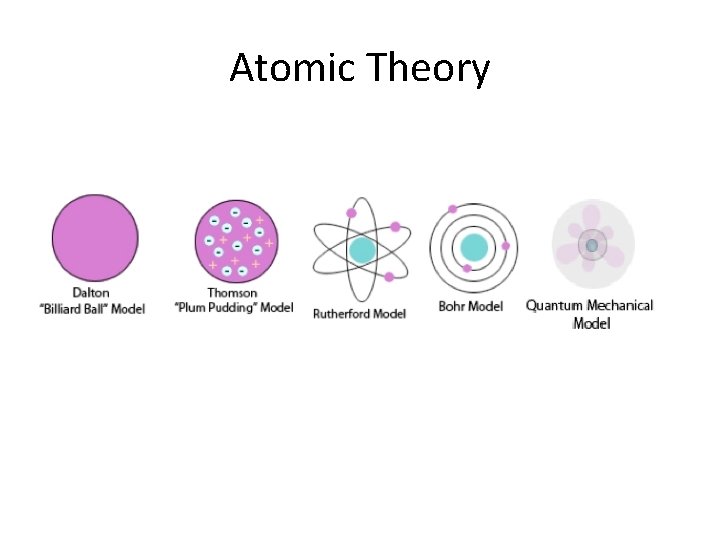
Atomic Theory

Atomic Theory • 400 BC Democritus – first concept of an atom – matter is made up of tiny particles, the properties of the particles determines the properties of the matter
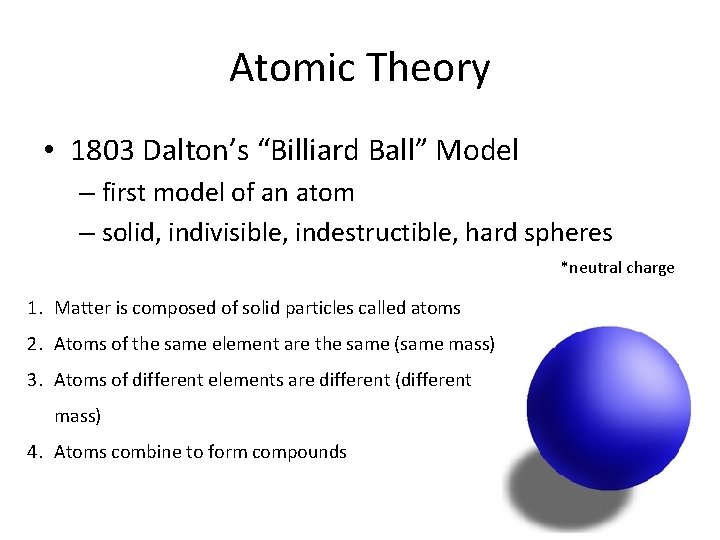
Atomic Theory • 1803 Dalton’s “Billiard Ball” Model – first model of an atom – solid, indivisible, indestructible, hard spheres *neutral charge 1. Matter is composed of solid particles called atoms 2. Atoms of the same element are the same (same mass) 3. Atoms of different elements are different (different mass) 4. Atoms combine to form compounds
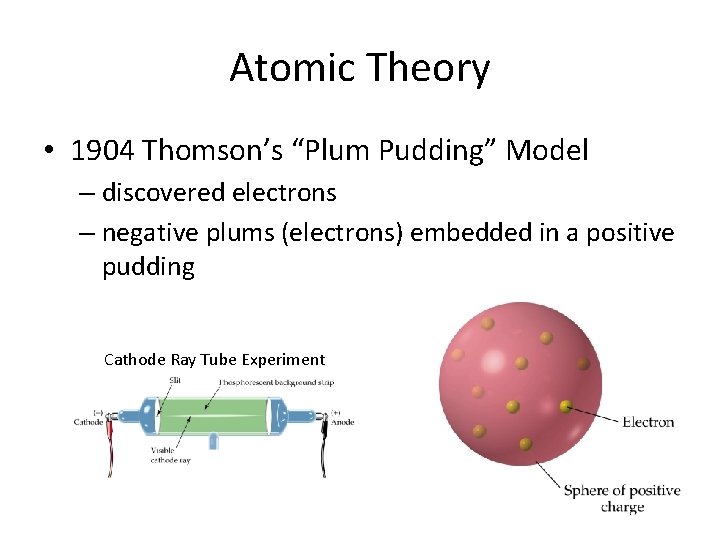
Atomic Theory • 1904 Thomson’s “Plum Pudding” Model – discovered electrons – negative plums (electrons) embedded in a positive pudding Cathode Ray Tube Experiment
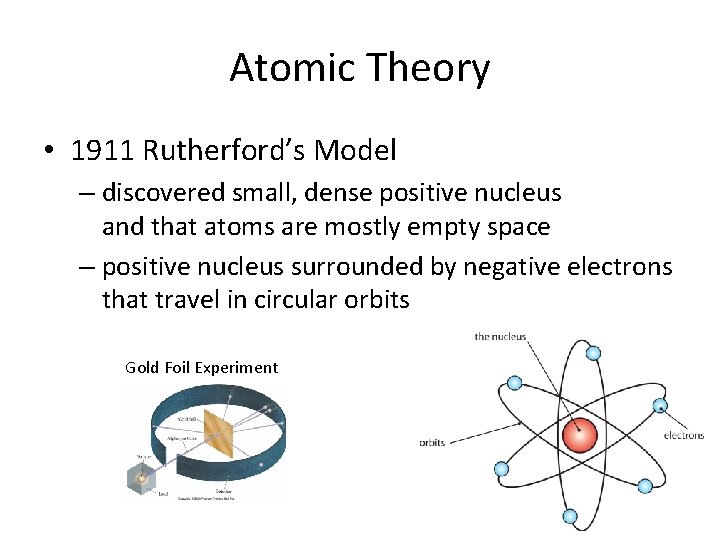
Atomic Theory • 1911 Rutherford’s Model – discovered small, dense positive nucleus and that atoms are mostly empty space – positive nucleus surrounded by negative electrons that travel in circular orbits Gold Foil Experiment
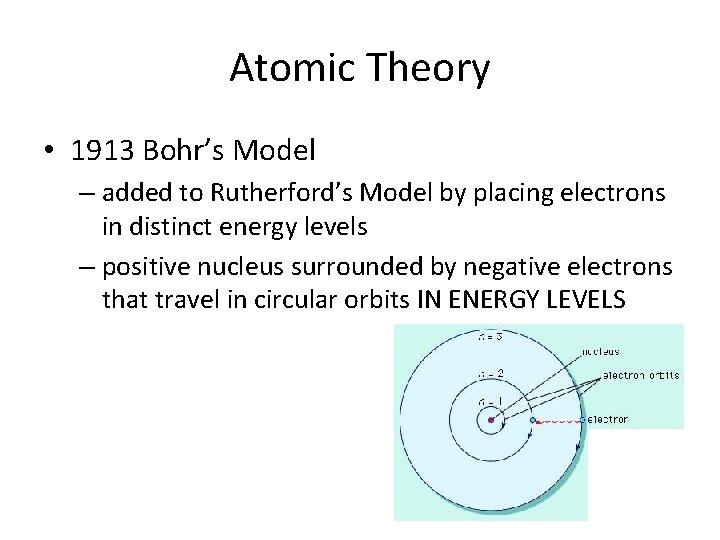
Atomic Theory • 1913 Bohr’s Model – added to Rutherford’s Model by placing electrons in distinct energy levels – positive nucleus surrounded by negative electrons that travel in circular orbits IN ENERGY LEVELS
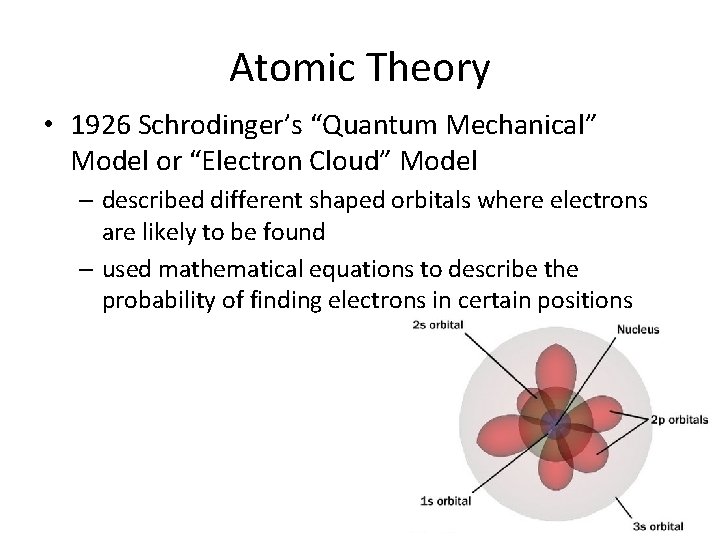
Atomic Theory • 1926 Schrodinger’s “Quantum Mechanical” Model or “Electron Cloud” Model – described different shaped orbitals where electrons are likely to be found – used mathematical equations to describe the probability of finding electrons in certain positions
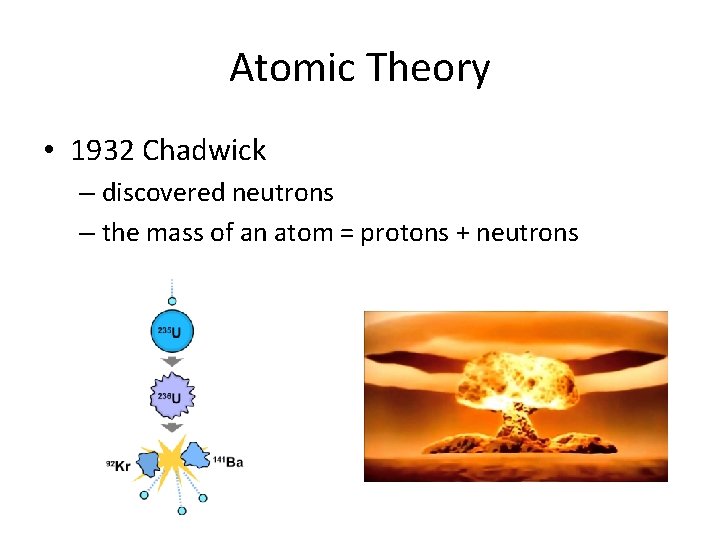
Atomic Theory • 1932 Chadwick – discovered neutrons – the mass of an atom = protons + neutrons
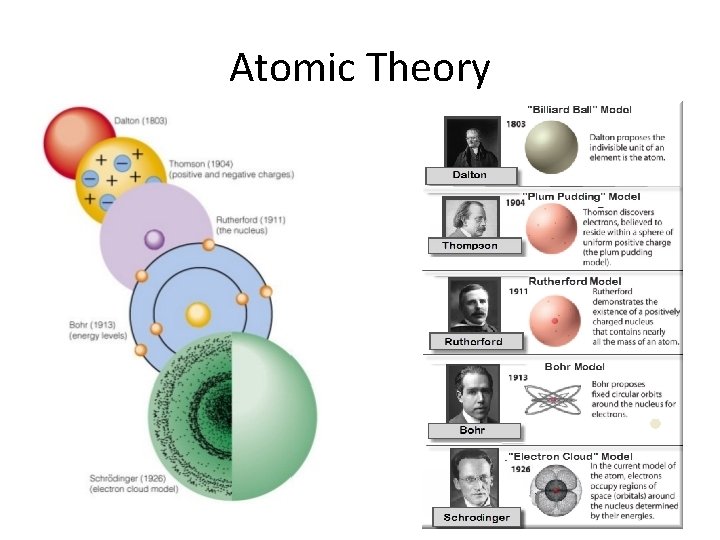
Atomic Theory

Conservation of Mass Lab • Conduct the following chemical reaction Ca. Cl 2 + Na 2 CO 3 2 Na. Cl + Ca. CO 3 – mass the reactants – conduct the reaction – separate the products – mass the products – compare the mass of the products with the mass of the reactants by calculating % yield and % error
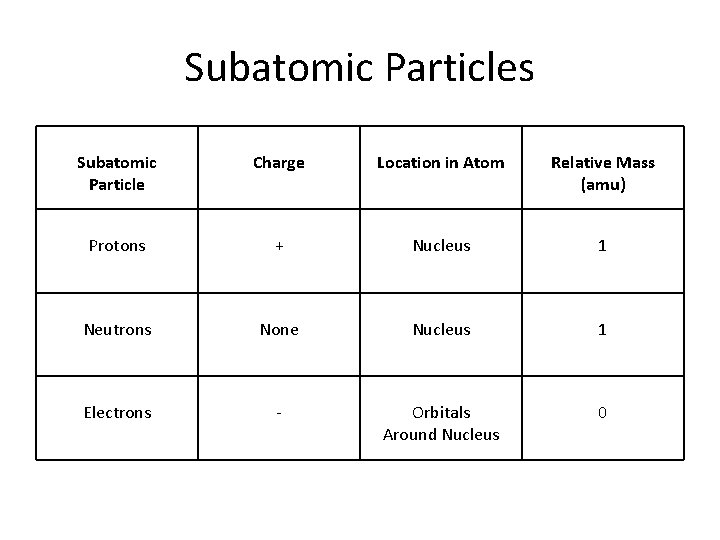
Subatomic Particles Subatomic Particle Charge Location in Atom Relative Mass (amu) Protons + Nucleus 1 Neutrons None Nucleus 1 Electrons - Orbitals Around Nucleus 0
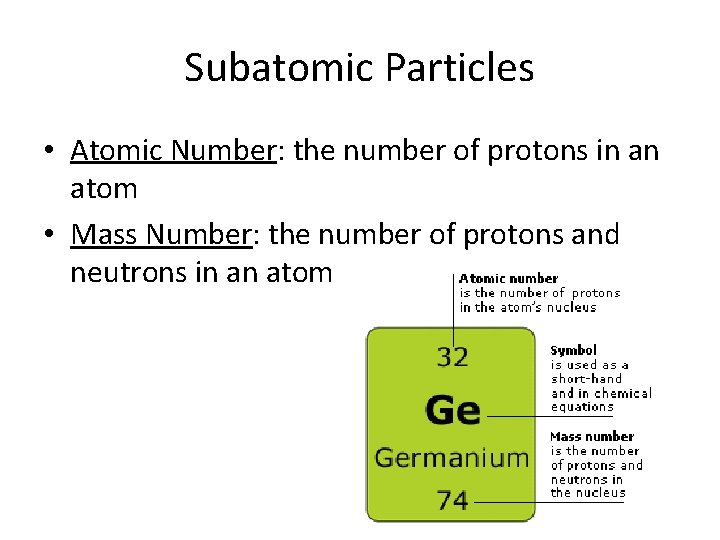
Subatomic Particles • Atomic Number: the number of protons in an atom • Mass Number: the number of protons and neutrons in an atom
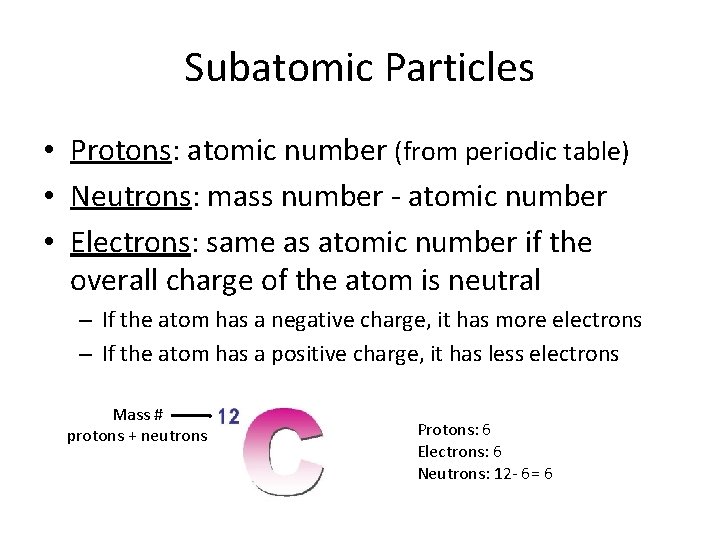
Subatomic Particles • Protons: atomic number (from periodic table) • Neutrons: mass number - atomic number • Electrons: same as atomic number if the overall charge of the atom is neutral – If the atom has a negative charge, it has more electrons – If the atom has a positive charge, it has less electrons Mass # protons + neutrons Protons: 6 Electrons: 6 Neutrons: 12 - 6= 6
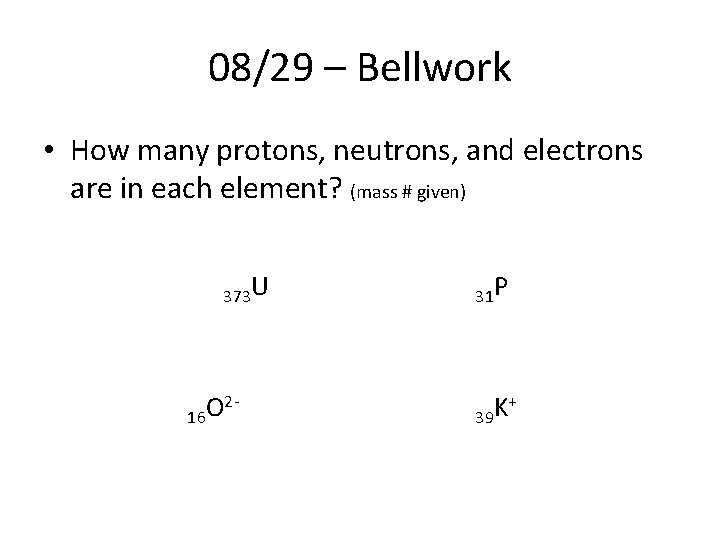
08/29 – Bellwork • How many protons, neutrons, and electrons are in each element? (mass # given) 373 U 2 O 16 31 P + K 39
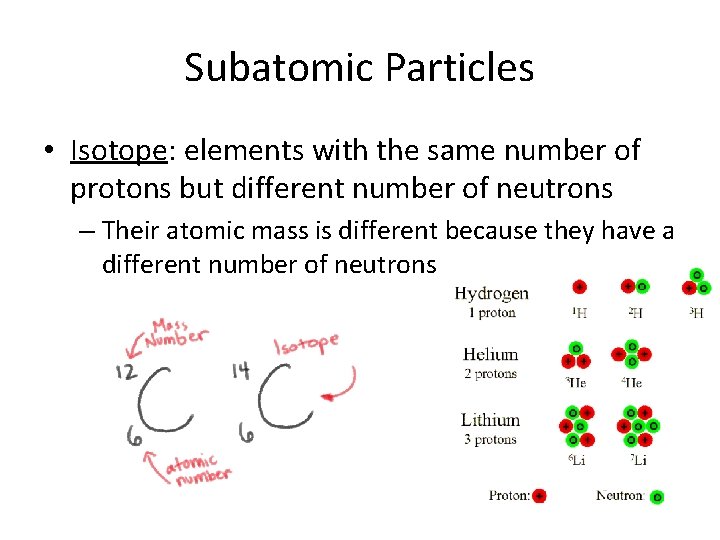
Subatomic Particles • Isotope: elements with the same number of protons but different number of neutrons – Their atomic mass is different because they have a different number of neutrons
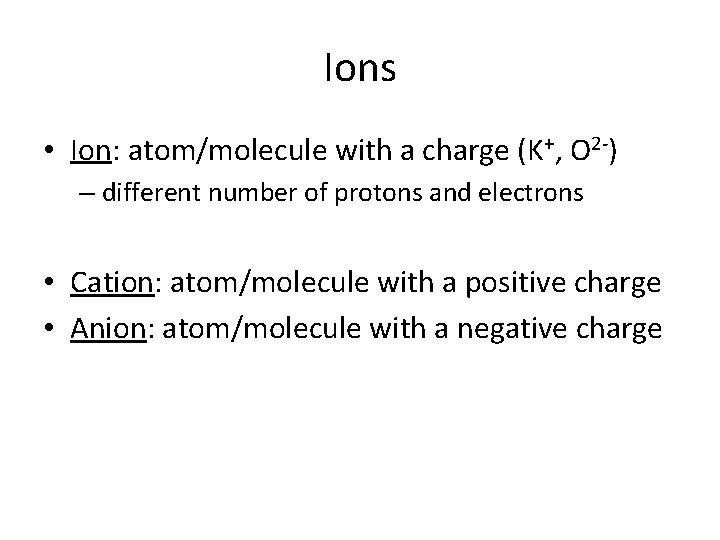
Ions • Ion: atom/molecule with a charge (K+, O 2 -) – different number of protons and electrons • Cation: atom/molecule with a positive charge • Anion: atom/molecule with a negative charge

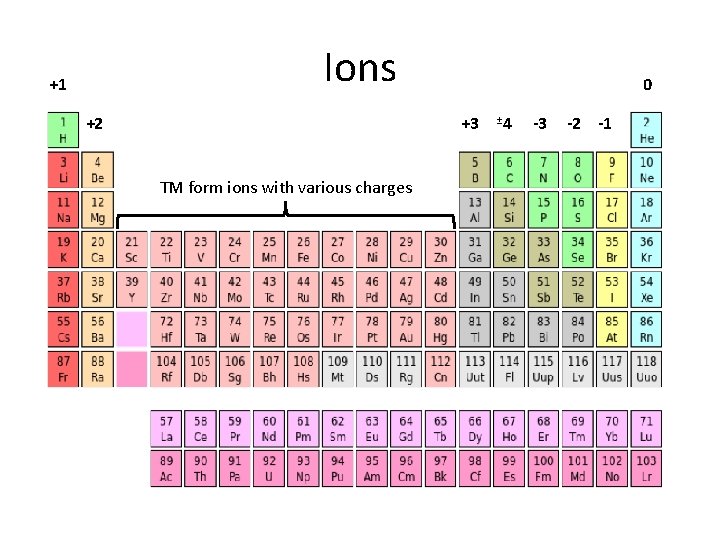
Ions +1 +2 0 +3 TM form ions with various charges ± 4 -3 -2 -1

Polyatomic Ions • Polyatomic: many atoms • Ion: charge Polyatomic Ion: many atoms stuck together with an overall charge
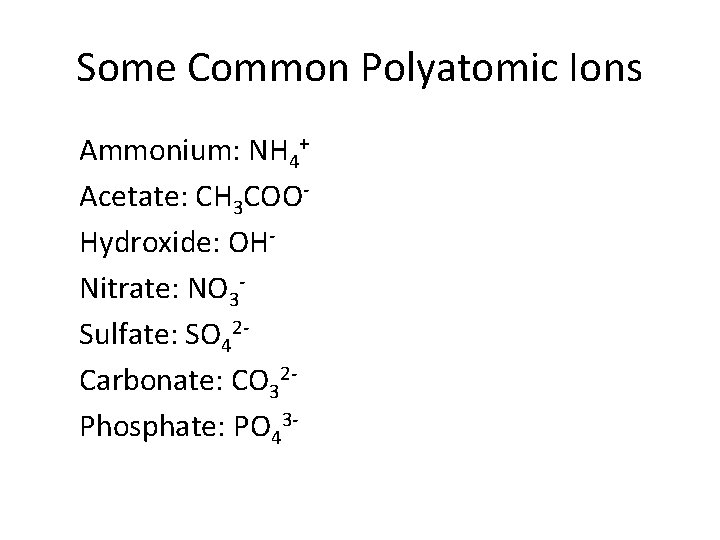
Some Common Polyatomic Ions Ammonium: NH 4+ Acetate: CH 3 COOHydroxide: OHNitrate: NO 3 Sulfate: SO 42 Carbonate: CO 32 Phosphate: PO 43 -

09/01 – Bellwork • Atomic Mass = 140 • # of e- = 58 (HINT: not an ion) • What is the Atomic # and number of protons? • What is the name of the element?
Compounds • Ionic Compounds (M/NM) • Covalent Compounds (NM/NM) • Metallic Compounds (M/M)
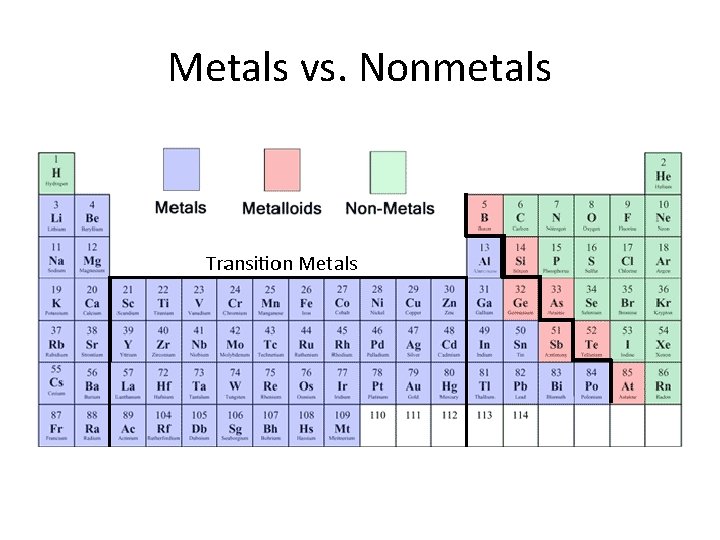
Metals vs. Nonmetals

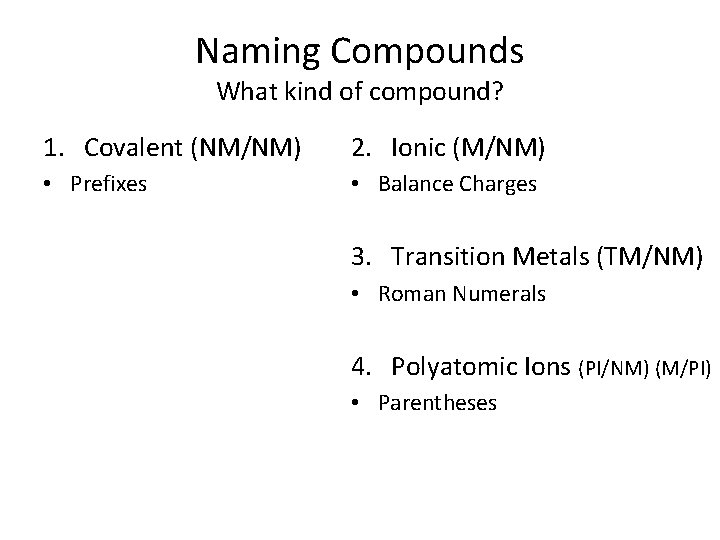
Naming Compounds What kind of compound? 1. Covalent (NM/NM) 2. Ionic (M/NM) 3. Transition Metals (TM/NM) 4. Polyatomic Ions (PI/NM) (M/PI)
Naming Compounds What kind of compound? 1. Covalent (NM/NM) 2. Ionic (M/NM) • Prefixes • Balance Charges 3. Transition Metals (TM/NM) • Roman Numerals 4. Polyatomic Ions (PI/NM) (M/PI) • Parentheses
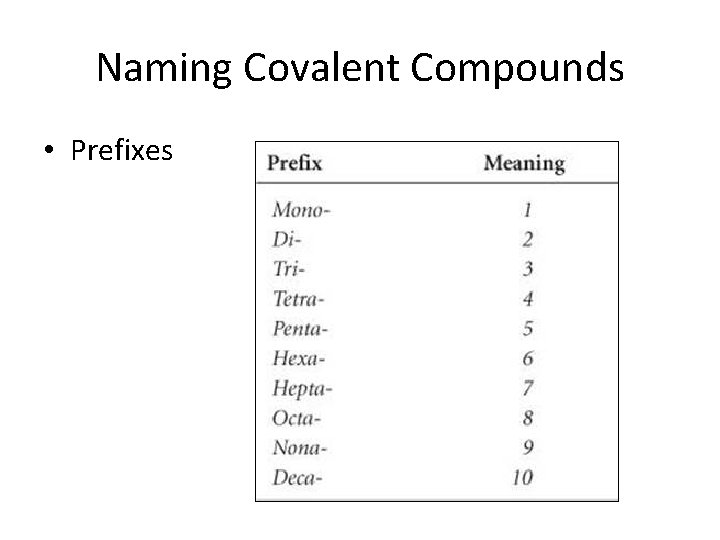
Naming Covalent Compounds • Prefixes
Naming Covalent Compounds • Formula to Words: 1. Write the name of each element 2. Give the second element an “ide” ending 3. Use prefixes to show many of each element (except mono on the first element) • Ex. – N 2 O 5 – PCl 5 – P 3 O 7 – CO 2

Naming Covalent Compounds • Words to Formula: 1. Write the symbol for each element 2. Add subscripts to show many of each element • Ex. – Nitrogen monoxide – Sulfur hexabromide – Dinitrogen tetroxide

Naming Ionic Compounds • Formula to Words: 1. Write the name of each element 2. Give the second element an “ide” ending • Ex: – Ca. Br 2 – K 2 O

Naming Ionic Compounds • Words to Formula: 1. Write the symbol for each element 2. Balance the charges to figure out the subscripts (Look at your periodic table for the charges) • Ex. – Magnesium fluoride – Aluminum sulfide – Calcium phosphide – Sodium Chloride – Aluminum bromide
Naming Transition Metals • TM have various positive charges – TM charges are written as roman numerals Copper (II) = Cu 2+ Iron (III) = Fe 3+ • Ex: – Manganese (IV) nitride – Copper (II) chloride – Iron (III) oxide – Cu 2 O – Fe. Cl 2
Polyatomic Ions • Treat as one ion! – Positive polyatomic ions = metals – Negative polyatomic ions = nonmetals • Ex: – Calcium carbonate – Cu. SO 4
Naming Compounds What kind of compound? 1. Covalent (NM/NM) 2. Ionic (M/NM) • Prefixes • Balance Charges 3. Transition Metals (TM/NM) • Roman Numerals 4. Polyatomic Ions (PI/NM) (M/PI) • Parentheses
- Slides: 41