Unit 2 Atomic Theory and Periodic Table Day

Unit 2: Atomic Theory and Periodic Table Day 12: Periodic Table and Reactivity Demonstrations
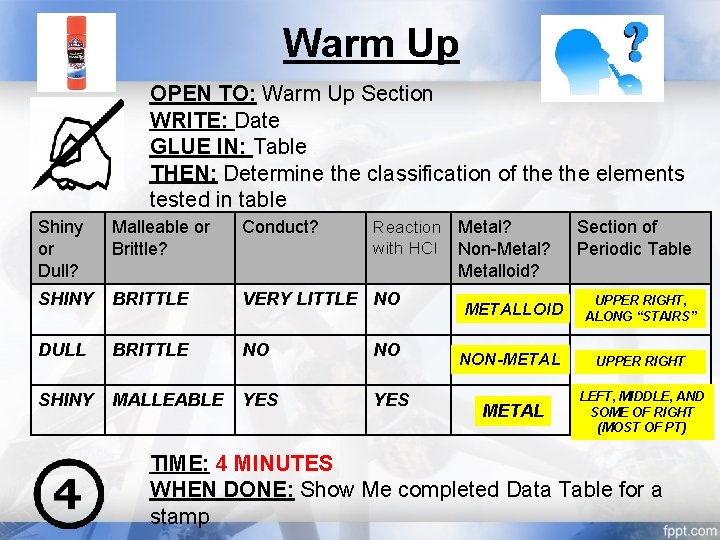
Warm Up OPEN TO: Warm Up Section WRITE: Date GLUE IN: Table THEN: Determine the classification of the elements tested in table Reaction Metal? with HCl Non-Metal? Metalloid? Shiny or Dull? Malleable or Brittle? Conduct? SHINY BRITTLE VERY LITTLE NO DULL BRITTLE NO NO SHINY MALLEABLE YES Section of Periodic Table METALLOID UPPER RIGHT, ALONG “STAIRS” NON-METAL UPPER RIGHT METAL LEFT, MIDDLE, AND SOME OF RIGHT (MOST OF PT) TIME: 4 MINUTES WHEN DONE: Show Me completed Data Table for a stamp

Agenda ONLY 4 MORE THURSDAY FLEX DAYS BEFORE FINALS! Ø Toolbox Entry Ø Color Code: Vocabulary of Groups Ø Properties of Groups Ø Reactivity of Metals: Group 1 Ø Demonstration: Lithium in Water

Long Term Learning Target I can use the periodic table to predict the relative reactivity of metals in groups 1, 2 and 3.
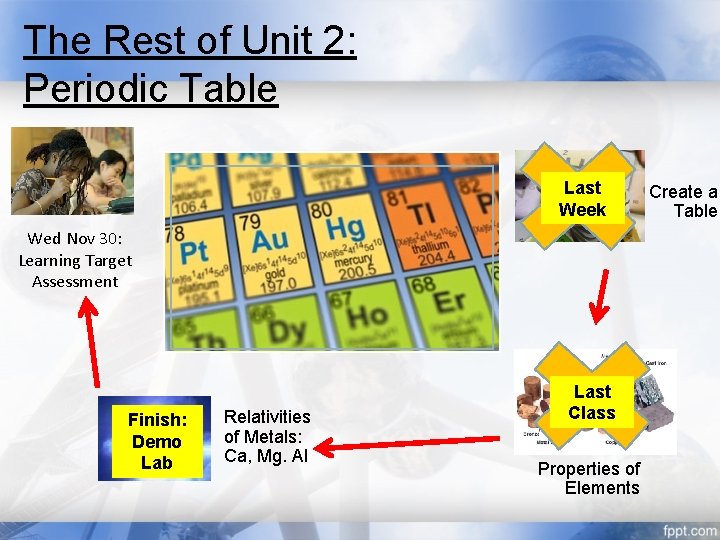
The Rest of Unit 2: Periodic Table Last Week Wed Nov 30: Learning Target Assessment Finish: Demo Lab Relativities of Metals: Ca, Mg. Al Last Class Properties of Elements Create a Table
Color Code Periodic Table FOLLOW ALONG: On Document Camera COLOR CODE: Groups on Periodic Table THEN: GLUE Periodic Table into Toolbox

Toolbox Entry GLUE: Color Coded Periodic Table and Table into Unit 2 Toolbox USING: Your knowledge, table partners, and lab analysis TRY TO FILL OUT AT LEAST THREE THINGS TIME: 3 MINUTES WHEN DONE: Say “hello” in as many languages as possible to your table partners
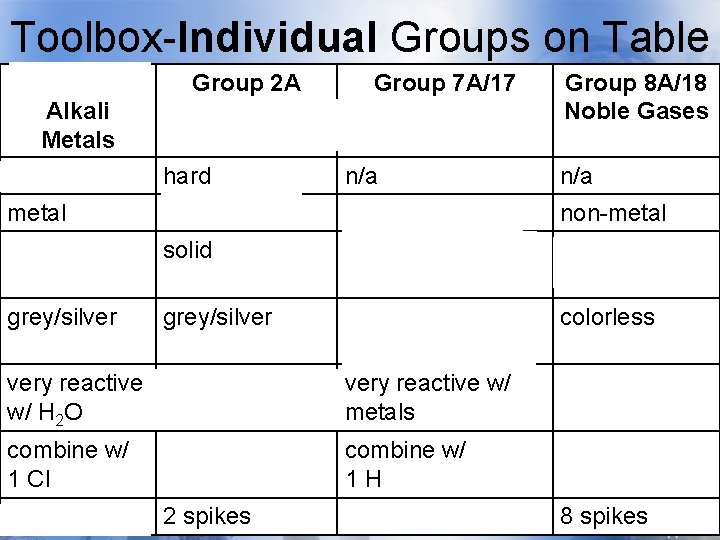
Toolbox-Individual Groups on Table Group 1 A Alkali Metals soft Group 2 A Group 7 A/17 Alkaline Earth Halogens Metals hard n/a Group 8 A/18 Noble Gases n/a metal non-metal solid gas, liquid, & solid gases grey/silver yellow/brown/blue (varies) colorless very reactive react with H 2 O w/ H 2 O very reactive w/ metals unreactive combine w/ 1 Cl combine w/ 2 Cl combine w/ 1 H n/a 1 spike 2 spikes 7 spikes 8 spikes
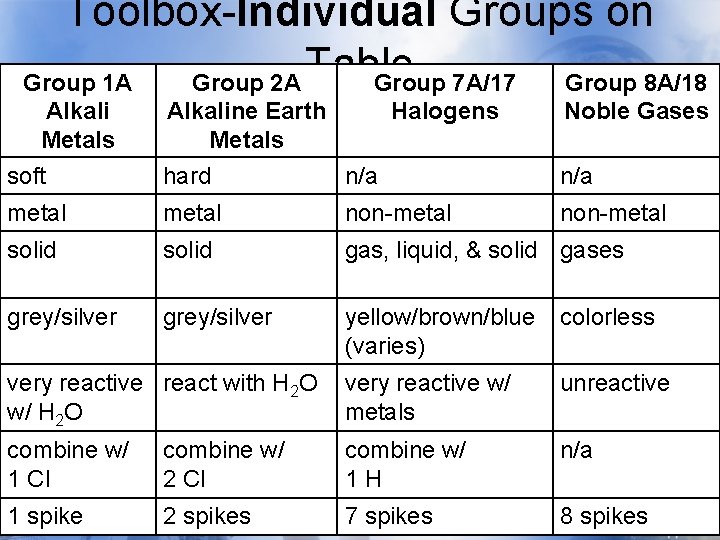
Toolbox-Individual Groups on Group 1 A Group 2 A Table Group 7 A/17 Group 8 A/18 Alkali Metals soft Alkaline Earth Halogens Metals hard n/a Noble Gases n/a metal non-metal solid gas, liquid, & solid gases grey/silver yellow/brown/blue (varies) colorless very reactive react with H 2 O w/ H 2 O very reactive w/ metals unreactive combine w/ 1 Cl combine w/ 2 Cl combine w/ 1 H n/a 1 spike 2 spikes 7 spikes 8 spikes
Set Up Cornell Notes SET UP: Cornell Note on Next Available Right-Hand page • Title: Reactivity of Metals: Part 1 • EQ: What is the trend of reactivity of metals in groups 1 (alkali metals)? Why? TIME: 1 MINUTE WHEN DONE: Be ready to take more notes and watch demonstration
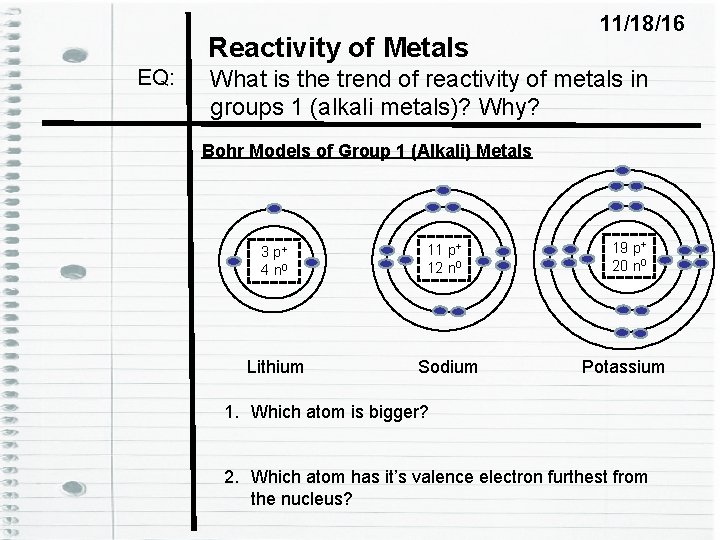
Reactivity of Metals EQ: 11/18/16 What is the trend of reactivity of metals in groups 1 (alkali metals)? Why? Bohr Models of Group 1 (Alkali) Metals 3 p+ 4 n 0 11 p+ 12 n 0 Lithium Sodium 19 p+ 20 n 0 Potassium 1. Which atom is bigger? 2. Which atom has it’s valence electron furthest from the nucleus?
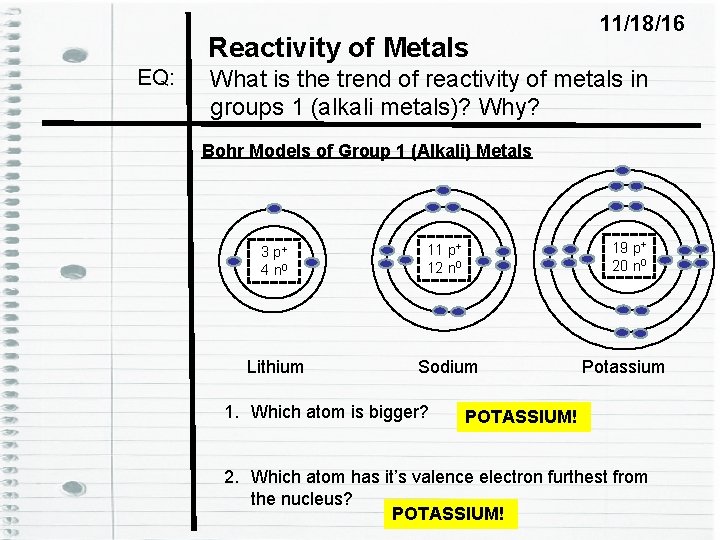
Reactivity of Metals EQ: 11/18/16 What is the trend of reactivity of metals in groups 1 (alkali metals)? Why? Bohr Models of Group 1 (Alkali) Metals 19 p+ 20 n 0 3 p+ 4 n 0 11 p+ 12 n 0 Lithium Sodium 1. Which atom is bigger? Potassium POTASSIUM! 2. Which atom has it’s valence electron furthest from the nucleus? POTASSIUM!
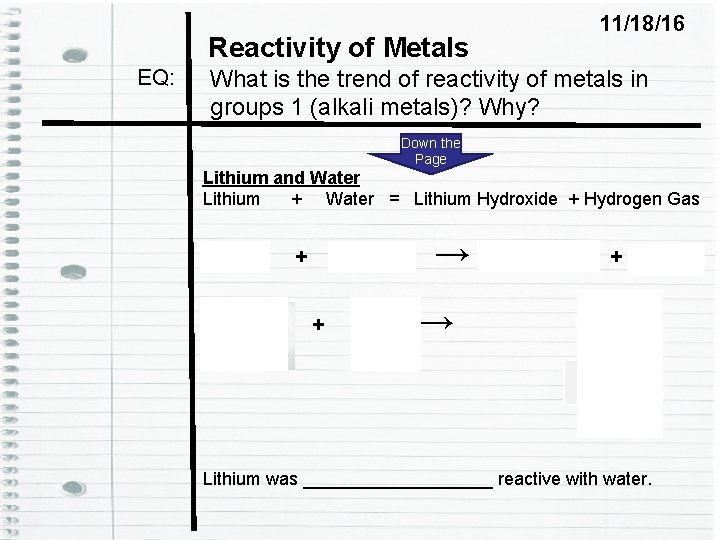
Reactivity of Metals EQ: 11/18/16 What is the trend of reactivity of metals in groups 1 (alkali metals)? Why? Down the Page Lithium and Water Lithium + Water = Lithium Hydroxide + Hydrogen Gas Li (s) + H 2 O (l) + → → Li. OH (aq) + H 2 (g) H 2 Li. OH Lithium was __________ reactive with water.
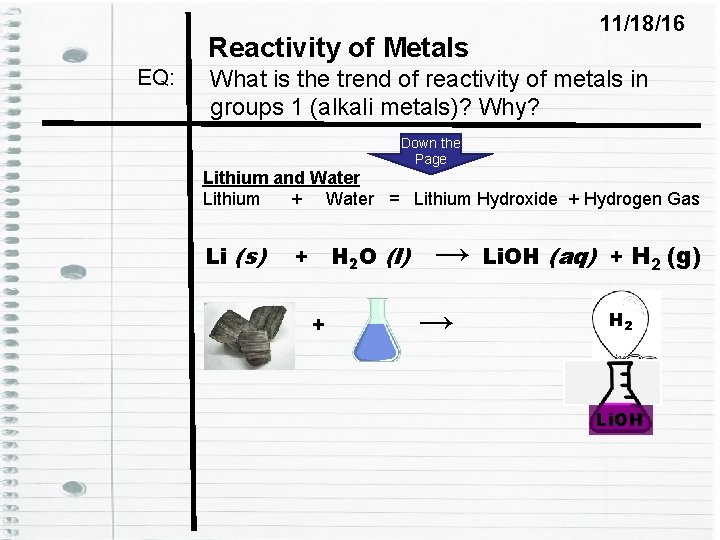
Reactivity of Metals EQ: 11/18/16 What is the trend of reactivity of metals in groups 1 (alkali metals)? Why? Down the Page Lithium and Water Lithium + Water = Lithium Hydroxide + Hydrogen Gas Li (s) + H 2 O (l) + → → Li. OH (aq) + H 2 (g) H 2 Li. OH
Reactivity of Metals EQ: 11/18/16 What is the trend of reactivity of metals in groups 1 (alkali metals)? Why? Down the Page At Least Two observations from Video 1. 2. Lithium was __________ reactive with water. Summary

Demo Lab: Activity of Metals READ: Introduction and Background ANNOTATE AND HIGHLIGHT: At least 3 things TIME: 8 MINUTES WHEN DONE: Classify each of the elements tested in demo lab (Group 1, 2, or 3)
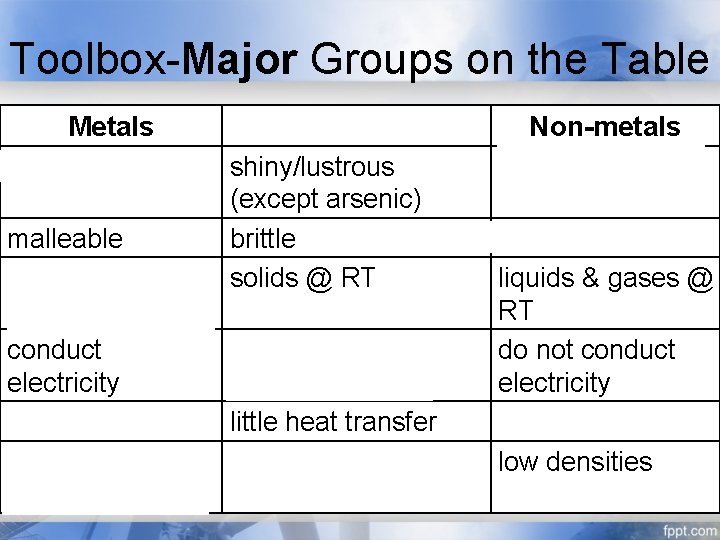
Toolbox-Major Groups on the Table Metals shiny/lustrous malleable solids @ RT (except mercury) conduct electricity conduct heat high densities Metalloids shiny/lustrous (except arsenic) brittle solids @ RT conduct little electricity little heat transfer intermediate densities Non-metals dull brittle if solid liquids & gases @ RT do not conduct electricity no heat transfer low densities
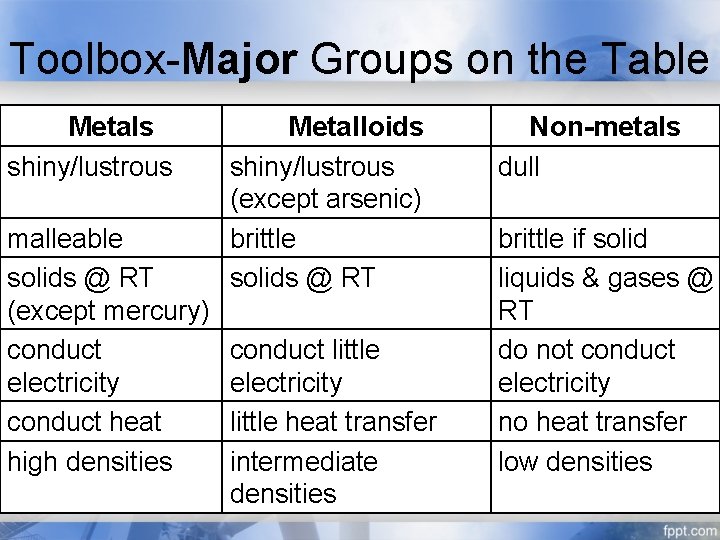
Toolbox-Major Groups on the Table Metals shiny/lustrous malleable solids @ RT (except mercury) conduct electricity conduct heat high densities Metalloids shiny/lustrous (except arsenic) brittle solids @ RT conduct little electricity little heat transfer intermediate densities Non-metals dull brittle if solid liquids & gases @ RT do not conduct electricity no heat transfer low densities
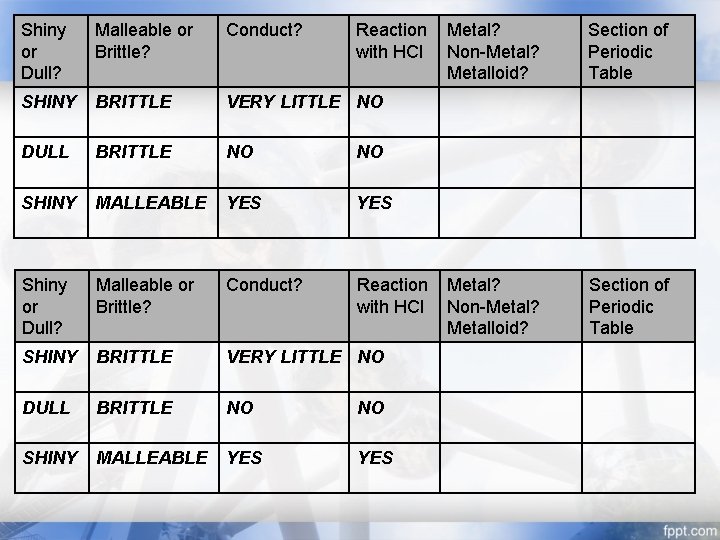
Shiny or Dull? Malleable or Brittle? Conduct? Reaction with HCl SHINY BRITTLE VERY LITTLE NO DULL BRITTLE NO NO SHINY MALLEABLE YES YES Metal? Non-Metal? Metalloid? Section of Periodic Table
- Slides: 19