Unit 2 Atomic Structure and the Periodic Table
Unit 2: Atomic Structure and the Periodic Table Trends
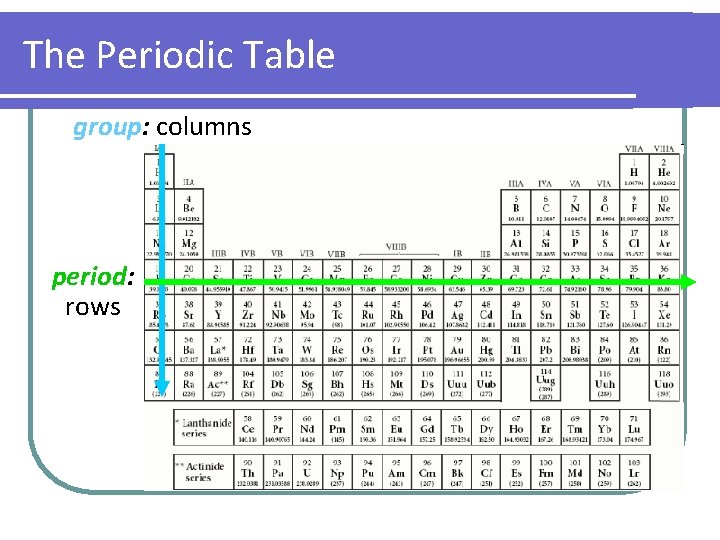
The Periodic Table group: columns period: rows
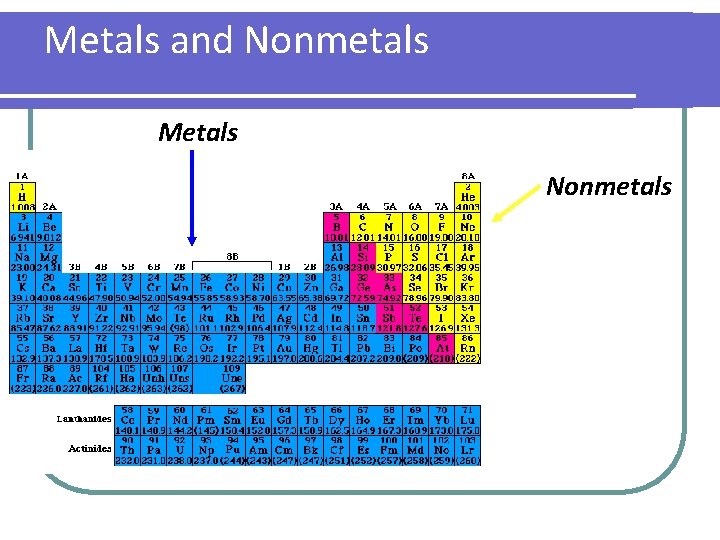
Metals and Nonmetals Metals Nonmetals
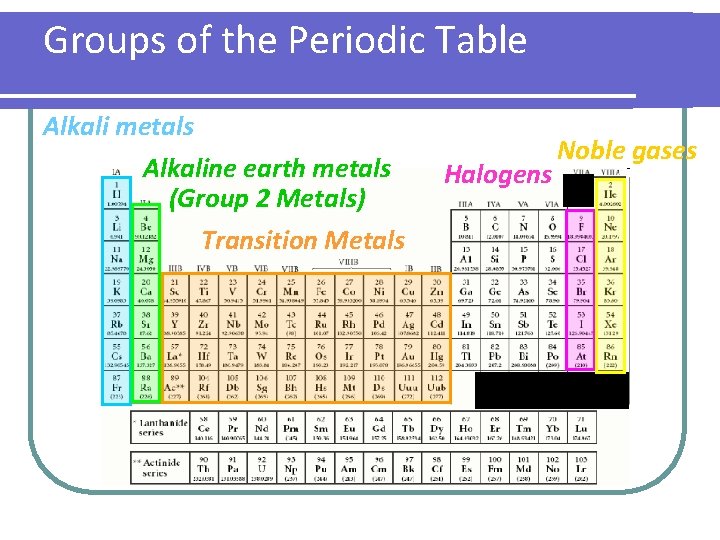
Groups of the Periodic Table Alkali metals Alkaline earth metals (Group 2 Metals) Transition Metals Halogens Noble gases
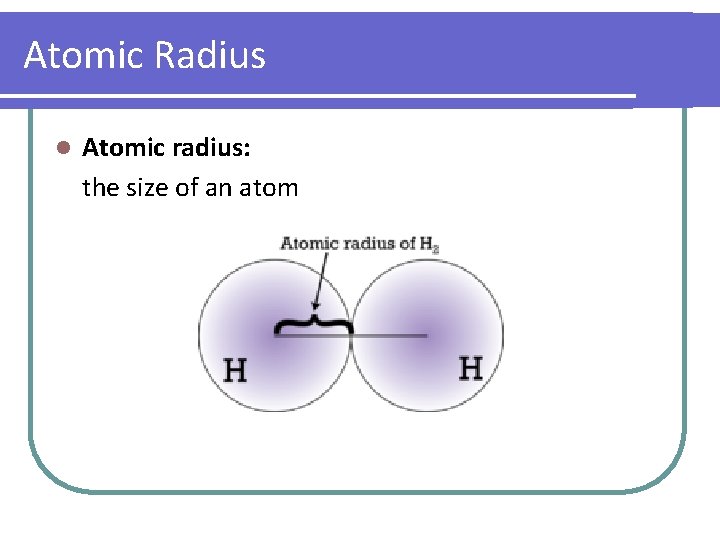
Atomic Radius l Atomic radius: the size of an atom
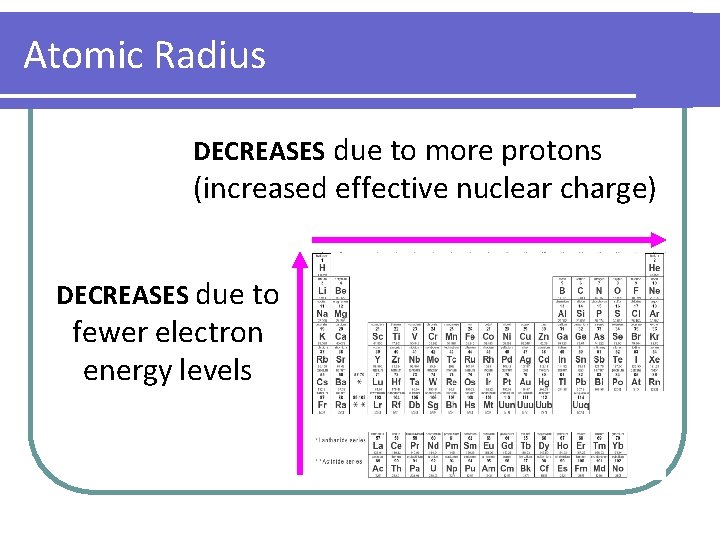
Atomic Radius DECREASES due to more protons (increased effective nuclear charge) DECREASES due to fewer electron energy levels
Ionization Energy l Ionization energy: The energy required to remove the outermost (highest energy) electron from a neutral atom in its ground state.
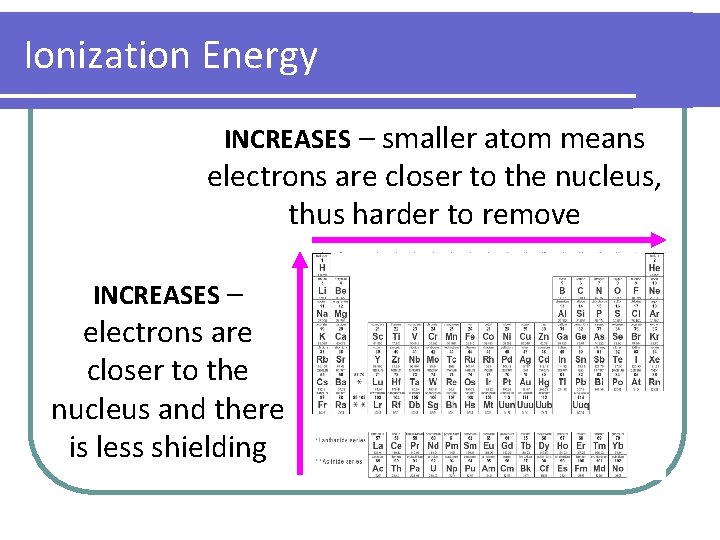
Ionization Energy INCREASES – smaller atom means electrons are closer to the nucleus, thus harder to remove INCREASES – electrons are closer to the nucleus and there is less shielding
Electronegativity l Electronegativity: ability of an atom in a molecule (covalent bond) to attract electrons
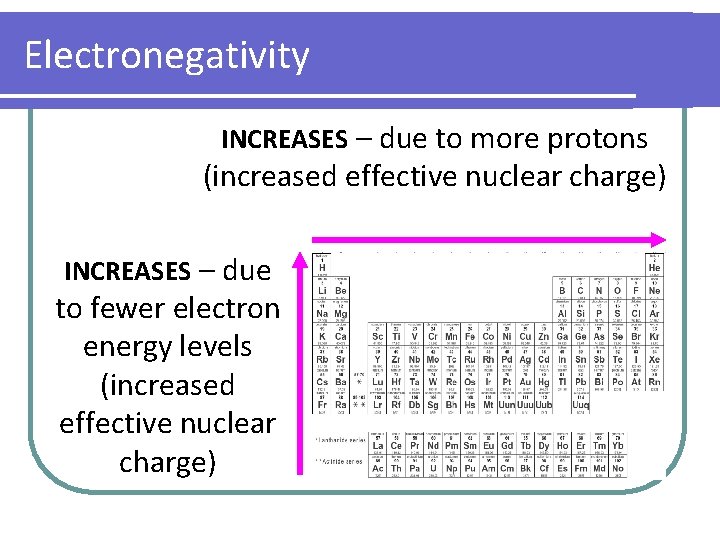
Electronegativity INCREASES – due to more protons (increased effective nuclear charge) INCREASES – due to fewer electron energy levels (increased effective nuclear charge)
Periodic Table Trends Assignment l ATOMIC RADIUS l l l IONIZATION ENERGY l l l read p. 259 -262 do p. 288: 7. 4, 7. 23 read p. 264 -268 do p. 290: 7. 41 (skip a), 7. 43, 7. 45 ELECTRONEGATIVITY l l read p. 308 do p. 334: 8. 36, 8. 37
- Slides: 11