Unit 14 Nuclear Chemistry Section 1 Its da



- Slides: 12

Unit 14: Nuclear Chemistry Section 1: “It’s da bomb!” – Fission and Fusion Reactions

Quick Review � Nucleus • Centermost part of an atom • Surrounded by electrons at different energy levels • Composed of protons and neutrons �Protons – Positive Charge �Neutrons – Neutral Charge �Electrons – Negative Charge

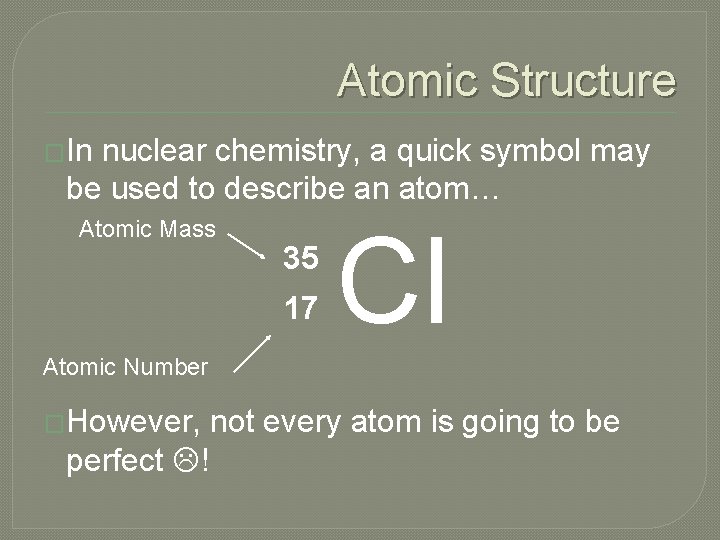
Atomic Structure �In nuclear chemistry, a quick symbol may be used to describe an atom… Atomic Mass 35 17 Cl Atomic Number �However, perfect ! not every atom is going to be

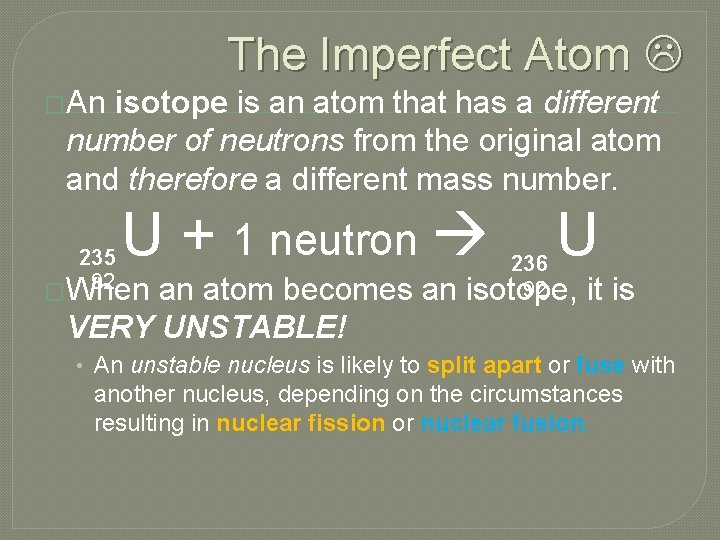
The Imperfect Atom �An isotope is an atom that has a different number of neutrons from the original atom and therefore a different mass number. U + 1 neutron 235 92 �When an atom becomes an VERY UNSTABLE! U 236 92 isotope, it is • An unstable nucleus is likely to split apart or fuse with another nucleus, depending on the circumstances resulting in nuclear fission or nuclear fusion.

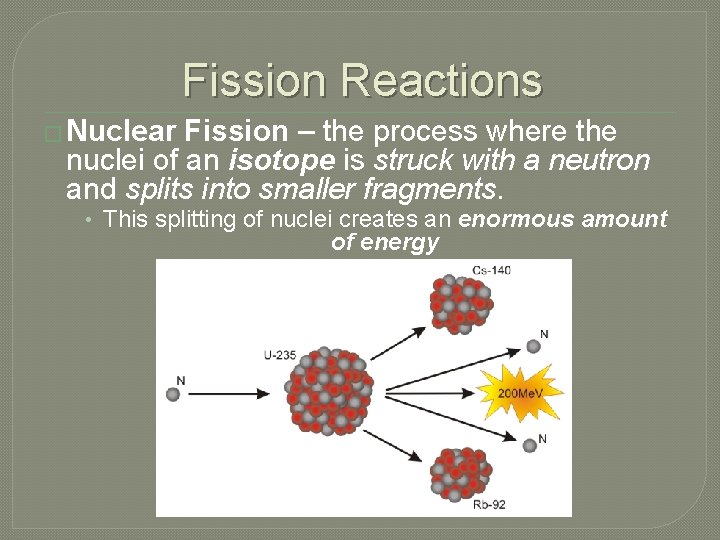
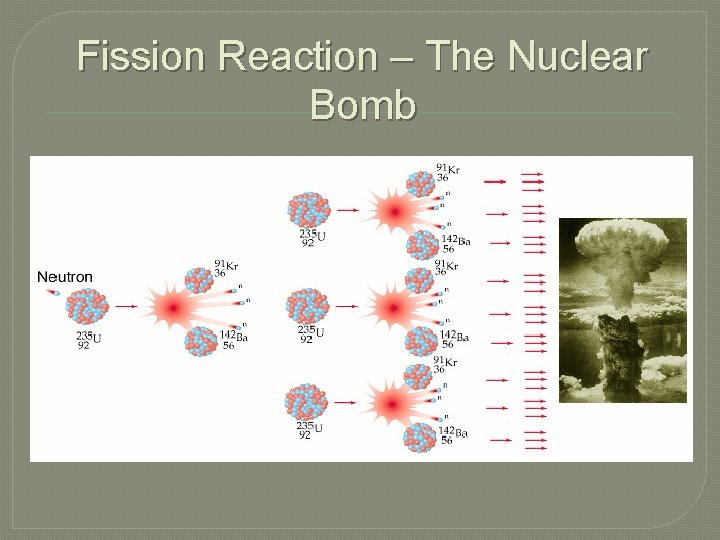
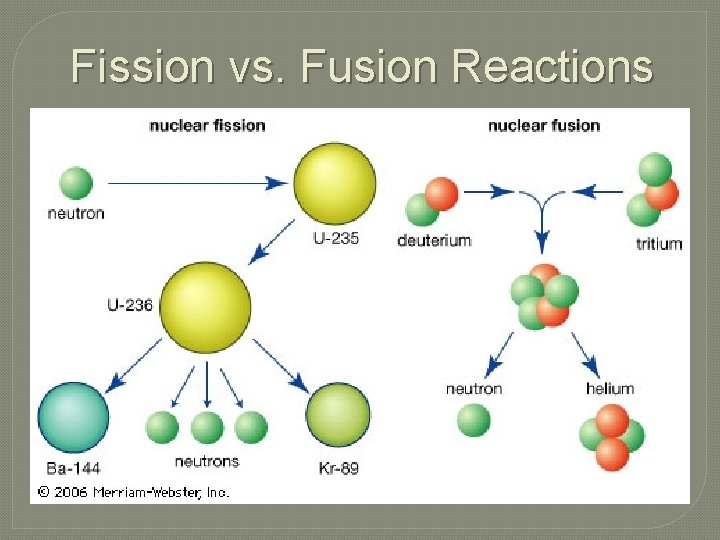
Fission Reactions � Nuclear Fission – the process where the nuclei of an isotope is struck with a neutron and splits into smaller fragments. • This splitting of nuclei creates an enormous amount of energy

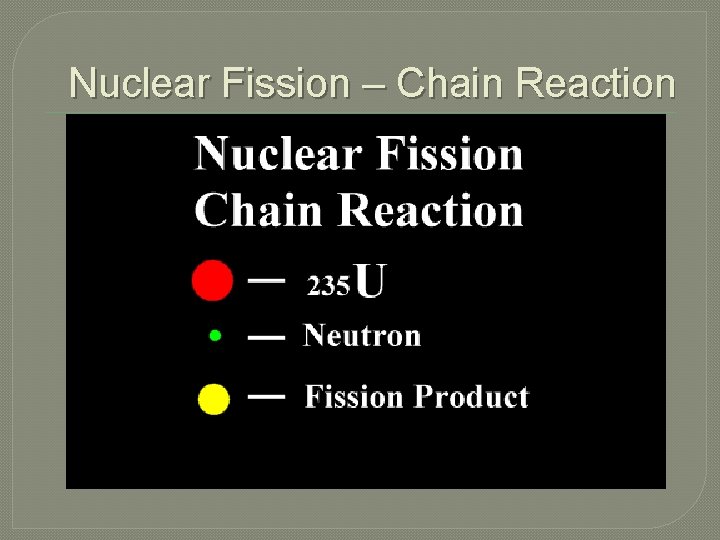
Nuclear Fission – Chain Reaction

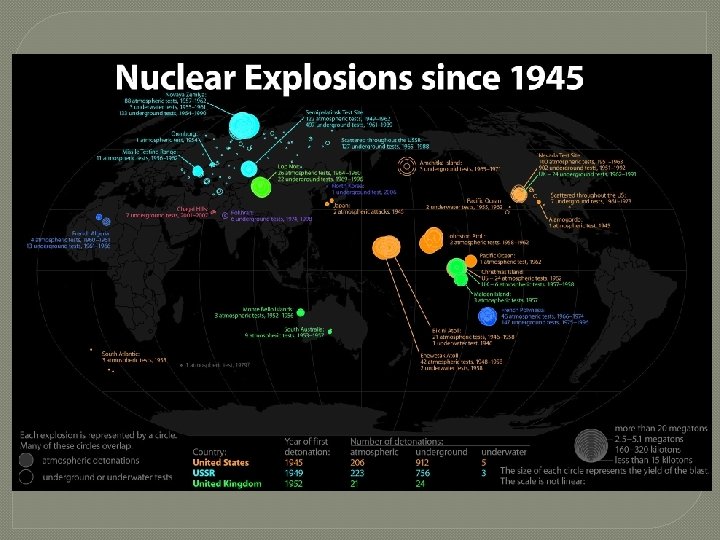
Fission Reaction – The Nuclear Bomb


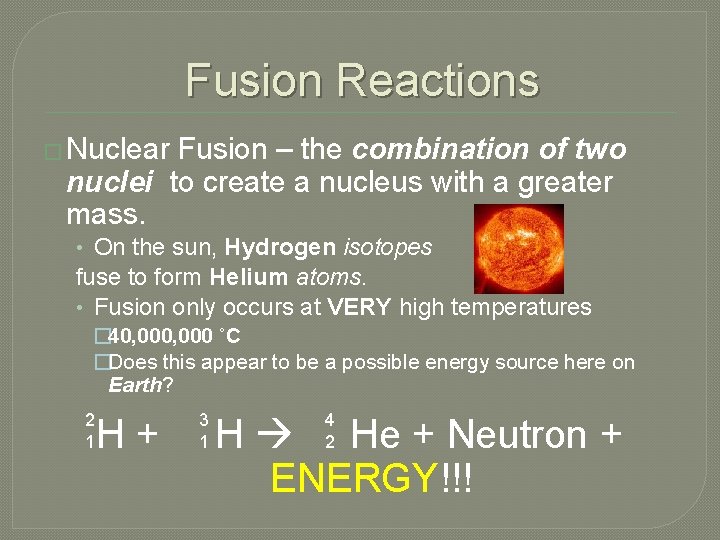
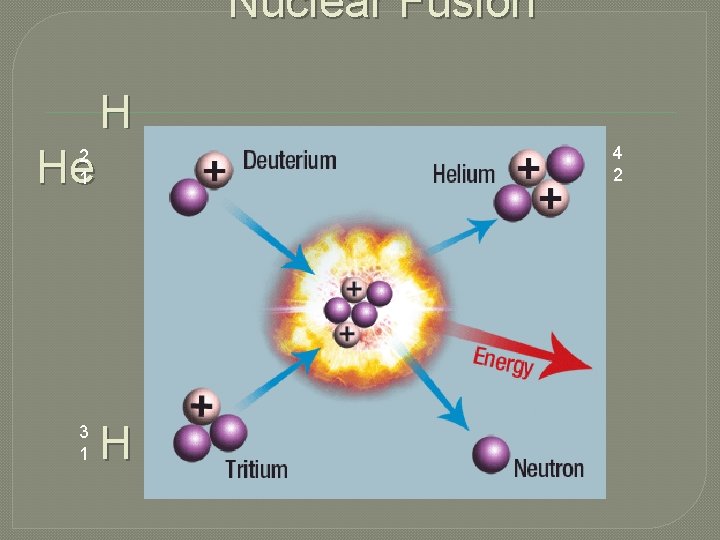
Fusion Reactions � Nuclear Fusion – the combination of two nuclei to create a nucleus with a greater mass. • On the sun, Hydrogen isotopes fuse to form Helium atoms. • Fusion only occurs at VERY high temperatures � 40, 000 ˚C �Does this appear to be a possible energy source here on Earth? 2 1 H+ 3 1 4 2 H He + Neutron + ENERGY!!!

Nuclear Fusion H He 4 2 2 1 3 1 H

Fission vs. Fusion Reactions

Summary � Summarize the idea of nuclear chemistry and the topics of fusion and fission. � Write in complete sentences… and remember you WILL have an audience for this writing (a partner) so think carefully about what you write!