Unit 11 Nuclear Chemistry Topic 1 Natural Radioactivity




- Slides: 26

Unit 11: Nuclear Chemistry Topic 1: Natural Radioactivity Objective: Identify the 4 modes of decay, use Table N and O to identify natural radioactive decay and write nuclear equations, identify the similarities and differences between physical, chemical, and nuclear reactions, identify what transmutation is

I. Natural Radioactivity and Stability § Nuclear Stability: the larger (more massive) a nucleus is, the harder it is for it to stay together radioactive § When a nucleus is ___________, it gives off decay from one element to another particles and changes ____________. This is known Natural decay or natural transmutation as ______________ § Atoms with an atomic number of 1 through 83 have at least one stable isotope, but… ____________________________ All isotopes of elements above 84 are more reactive and are natural radioisotopes

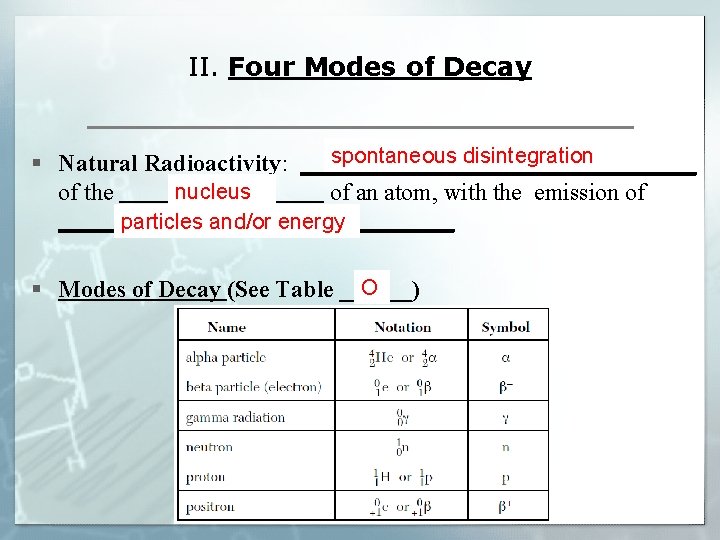
II. Four Modes of Decay spontaneous disintegration § Natural Radioactivity: _________________ nucleus of the _________ of an atom, with the emission of _________________ particles and/or energy O § Modes of Decay (See Table ______)

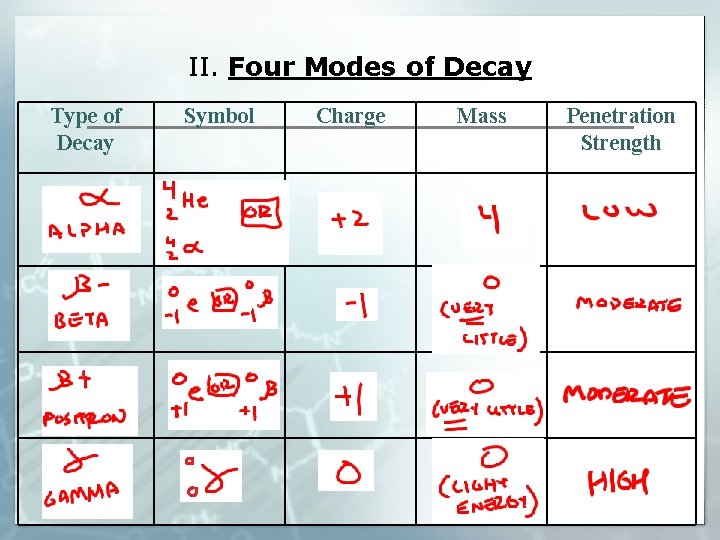
II. Four Modes of Decay Type of Decay Symbol Charge Mass Penetration Strength

II. Four Modes of Decay § Transmutations: When a nucleus decays into a new and different nucleus (also called radioactive decay) § Penetration: How far into a material the radioactive particle will go

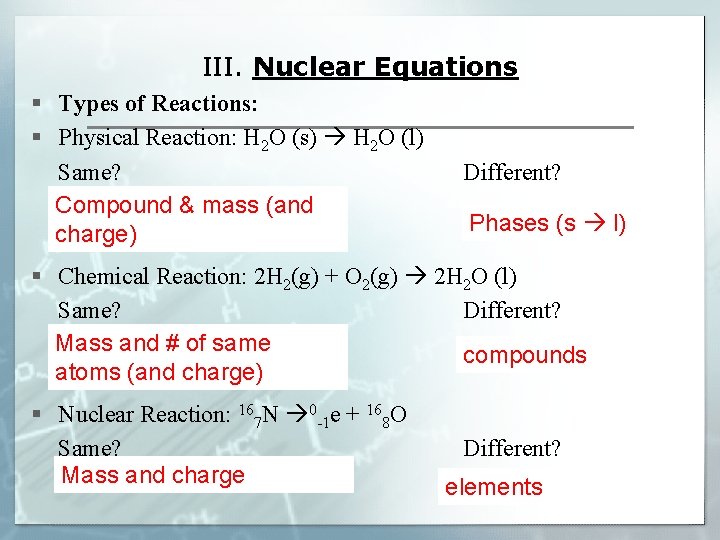
III. Nuclear Equations § Types of Reactions: § Physical Reaction: H 2 O (s) H 2 O (l) Same? Compound & mass (and charge) Different? Phases (s l) § Chemical Reaction: 2 H 2(g) + O 2(g) 2 H 2 O (l) Same? Different? Mass and # of same compounds atoms (and charge) § Nuclear Reaction: 167 N 0 -1 e + 168 O Same? Mass and charge Different? elements

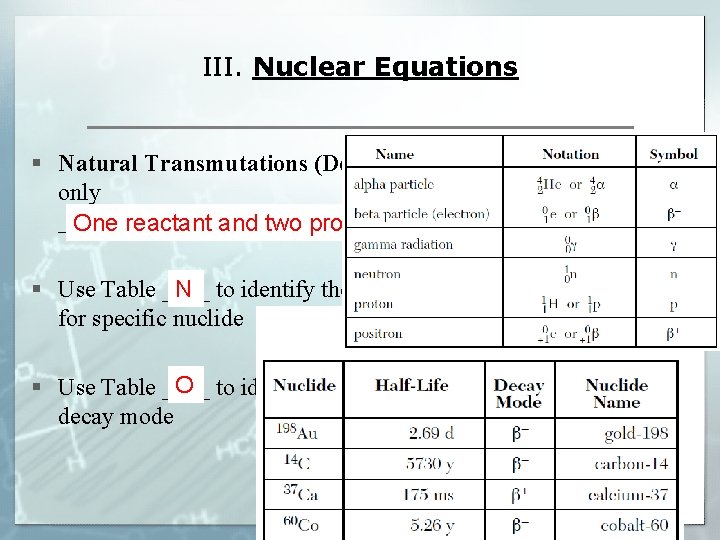
III. Nuclear Equations § Natural Transmutations (Decay) always has only __________________ One reactant and two products N § Use Table ____ to identify the type of decay for specific nuclide O § Use Table ____ to identify notation of each decay mode

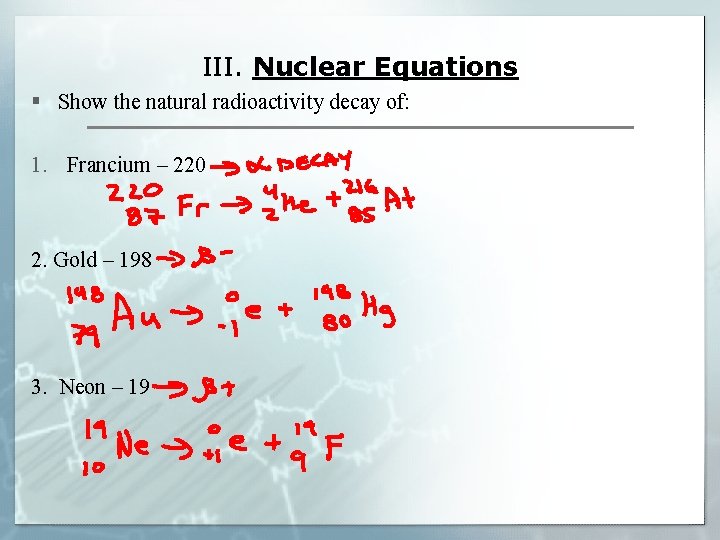
III. Nuclear Equations § Show the natural radioactivity decay of: 1. Francium – 220 2. Gold – 198 3. Neon – 19

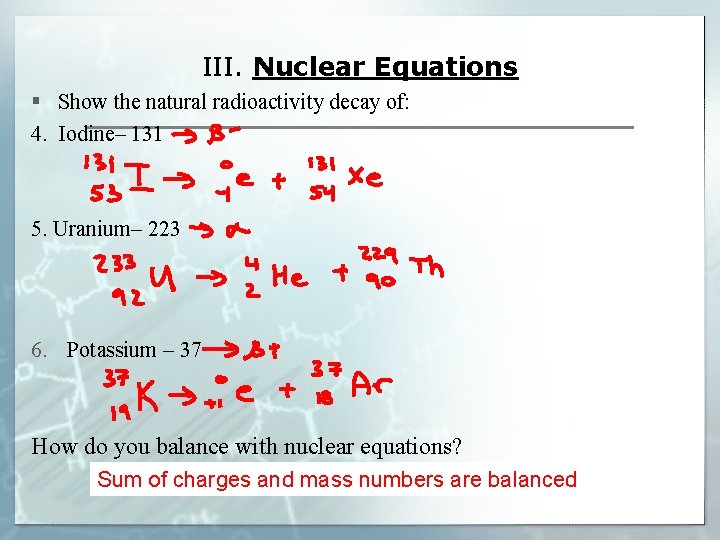
III. Nuclear Equations § Show the natural radioactivity decay of: 4. Iodine– 131 5. Uranium– 223 6. Potassium – 37 How do you balance with nuclear equations? Sum of charges and mass numbers are balanced

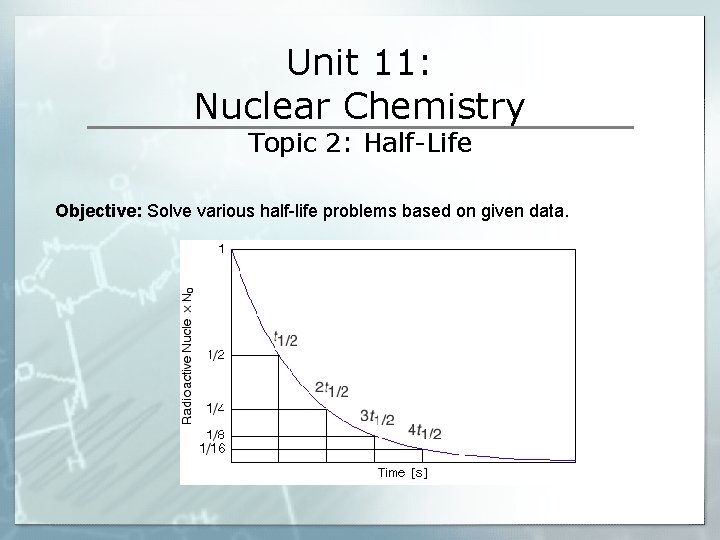
Unit 11: Nuclear Chemistry Topic 2: Half-Life Objective: Solve various half-life problems based on given data.

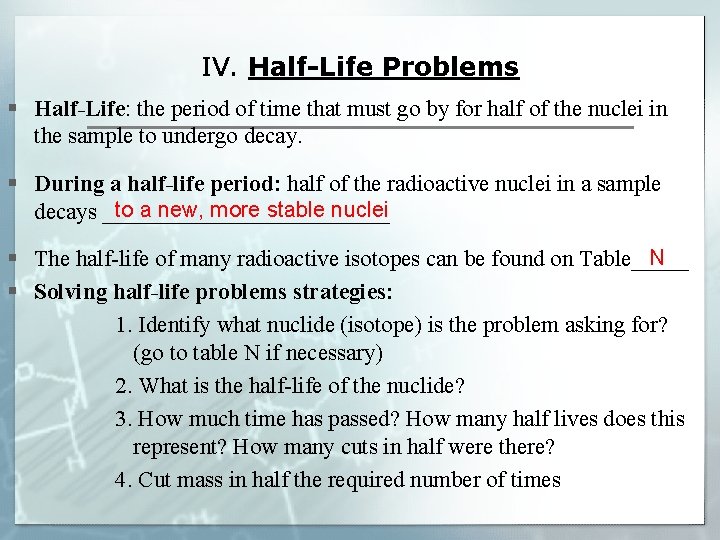
IV. Half-Life Problems § Half-Life: the period of time that must go by for half of the nuclei in the sample to undergo decay. § During a half-life period: half of the radioactive nuclei in a sample to a new, more stable nuclei decays _____________ N § The half-life of many radioactive isotopes can be found on Table_____ § Solving half-life problems strategies: 1. Identify what nuclide (isotope) is the problem asking for? (go to table N if necessary) 2. What is the half-life of the nuclide? 3. How much time has passed? How many half lives does this represent? How many cuts in half were there? 4. Cut mass in half the required number of times

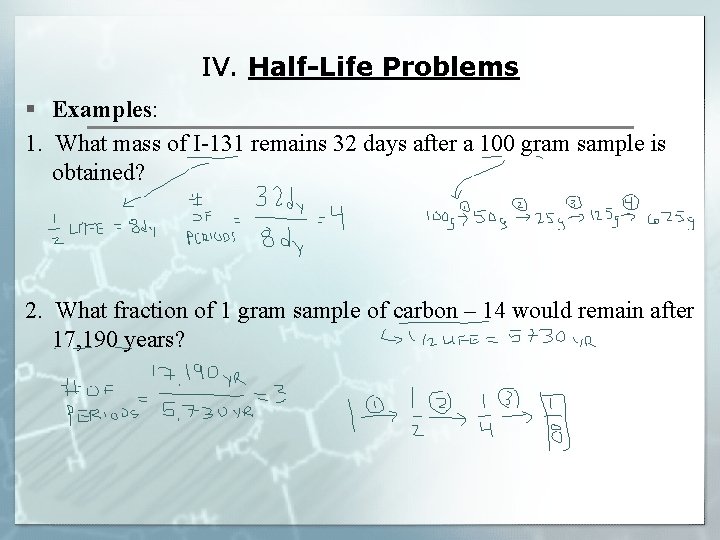
IV. Half-Life Problems § Examples: 1. What mass of I-131 remains 32 days after a 100 gram sample is obtained? 2. What fraction of 1 gram sample of carbon – 14 would remain after 17, 190 years?

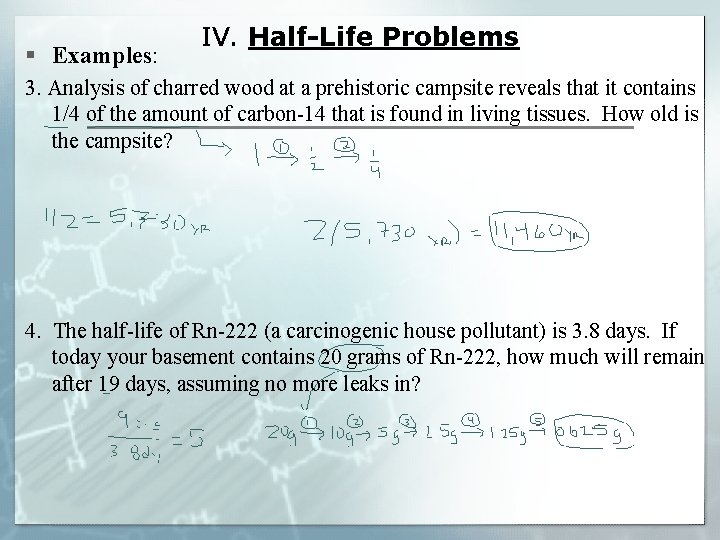
§ Examples: IV. Half-Life Problems 3. Analysis of charred wood at a prehistoric campsite reveals that it contains 1/4 of the amount of carbon-14 that is found in living tissues. How old is the campsite? 4. The half-life of Rn-222 (a carcinogenic house pollutant) is 3. 8 days. If today your basement contains 20 grams of Rn-222, how much will remain after 19 days, assuming no more leaks in?

§ Examples: IV. Half-Life Problems 5. The half-life of Tc-99 m* (used to locate brain tumors) is 6. 0 hours. If 10. Micrograms are left after 24 hours, how much Tc-99 m was administered? 6. A radioactive sample is placed next to a Geiger counter and monitored. In 20. 0 hours, the counter’s reading goes from 500 counts/minute to 125 counts/minute. How long is the half-life?

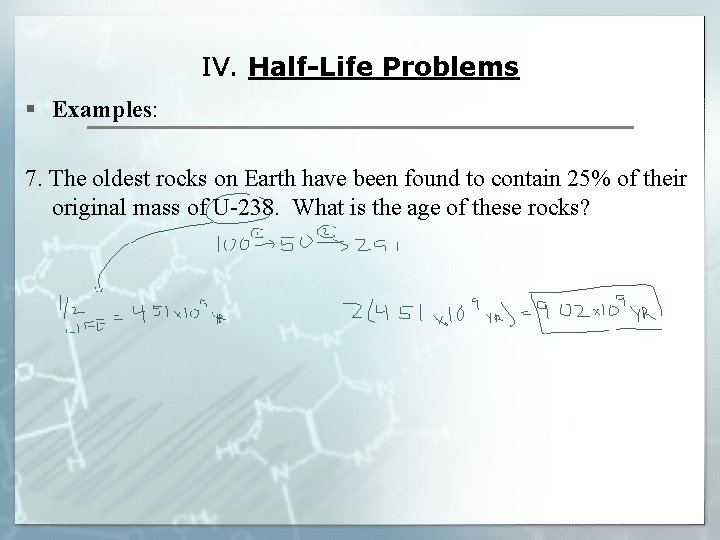
IV. Half-Life Problems § Examples: 7. The oldest rocks on Earth have been found to contain 25% of their original mass of U-238. What is the age of these rocks?

Unit 11: Nuclear Chemistry Topic 3: Artificial Transmutations Objective: Write artificial transmutation reactions, identify the similarities and differences between artificial/natural decay, and identify the similarities and differences fission/fusion reactions.

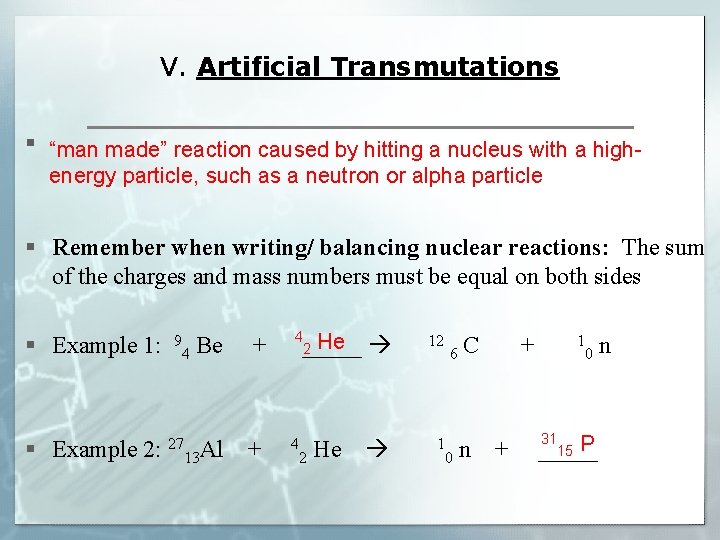
V. Artificial Transmutations § “man made” reaction caused by hitting a nucleus with a highenergy particle, such as a neutron or alpha particle § Remember when writing/ balancing nuclear reactions: The sum of the charges and mass numbers must be equal on both sides 4 He 12 C + 1 n § Example 1: 94 Be + _____ 2 6 0 31 15 P § Example 2: 2713 Al + 42 He 10 n + _____

V. Artificial Transmutations PRACTICE 1 – 5 on the front of Worksheet 3

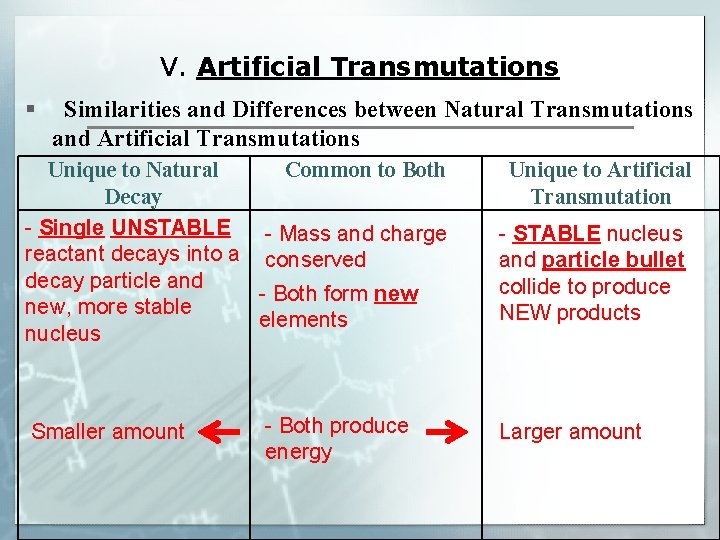
V. Artificial Transmutations § Similarities and Differences between Natural Transmutations and Artificial Transmutations Unique to Natural Common to Both Decay - Single UNSTABLE - Mass and charge reactant decays into a conserved decay particle and - Both form new, more stable elements nucleus Smaller amount - Both produce energy Unique to Artificial Transmutation - STABLE nucleus and particle bullet collide to produce NEW products Larger amount

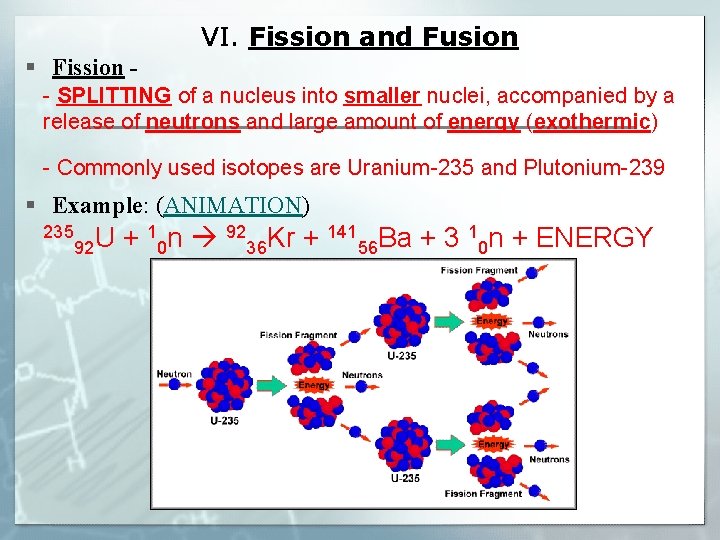
VI. Fission and Fusion § Fission - - SPLITTING of a nucleus into smaller nuclei, accompanied by a release of neutrons and large amount of energy (exothermic) - Commonly used isotopes are Uranium-235 and Plutonium-239 § Example: (ANIMATION) 235 1 n 92 Kr + 141 Ba + 3 1 n + ENERGY U + 92 0 36 56 0

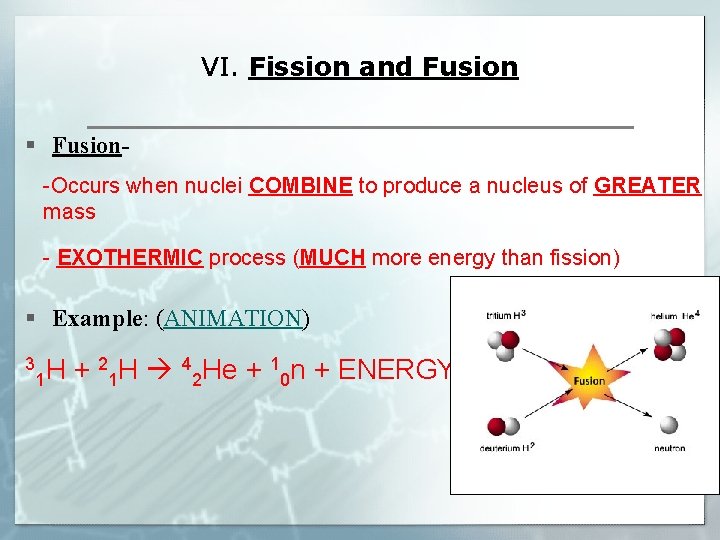
VI. Fission and Fusion § Fusion- -Occurs when nuclei COMBINE to produce a nucleus of GREATER mass - EXOTHERMIC process (MUCH more energy than fission) § Example: (ANIMATION) 3 2 H 4 He + 1 n + ENERGY H + 1 1 2 0

VII. Where does Energy come from? -Both reactions sacrifice nuclear mass to form energy (E= mc 2) - the “missing mass” in a nuclear reaction is called the mass defect and is the energy released in the reaction VIDEO How are nuclear power plant works?

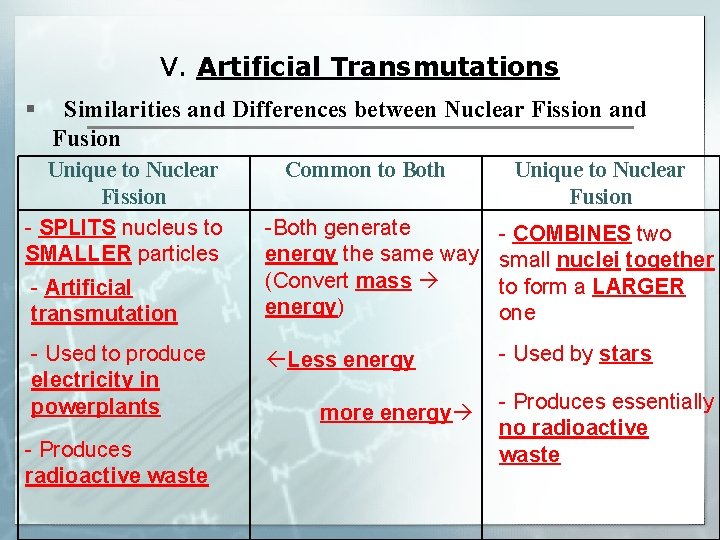
V. Artificial Transmutations § Similarities and Differences between Nuclear Fission and Fusion Unique to Nuclear Fission - SPLITS nucleus to SMALLER particles - Artificial transmutation - Used to produce electricity in powerplants - Produces radioactive waste Common to Both Unique to Nuclear Fusion -Both generate energy the same way (Convert mass energy) - COMBINES two small nuclei together to form a LARGER one ßLess energy - Used by stars more energy - Produces essentially no radioactive waste

Unit 11: Nuclear Chemistry Topic 4: Uses and Dangers of Radioisotopes Objective: Understand the benefits and risks of nuclear reactions, identify specific uses of common isotopes

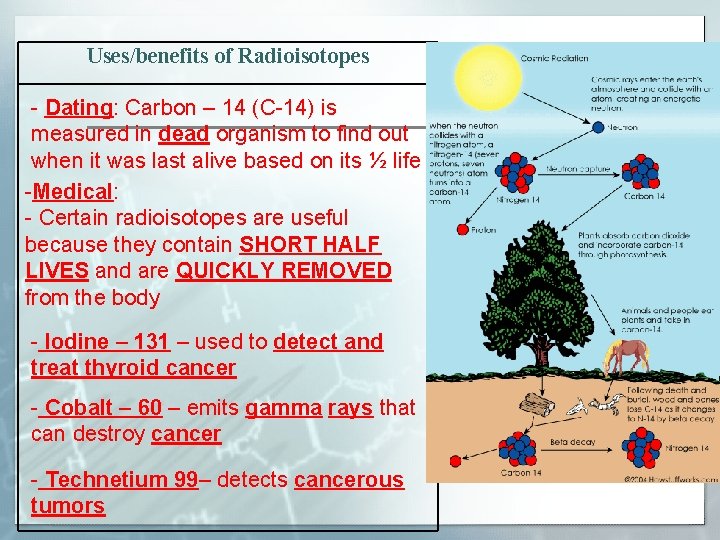
Uses/benefits of Radioisotopes - Dating: Carbon – 14 (C-14) is measured in dead organism to find out when it was last alive based on its ½ life -Medical: - Certain radioisotopes are useful because they contain SHORT HALF LIVES and are QUICKLY REMOVED from the body - Iodine – 131 – used to detect and treat thyroid cancer - Cobalt – 60 – emits gamma rays that can destroy cancer - Technetium 99– detects cancerous tumors

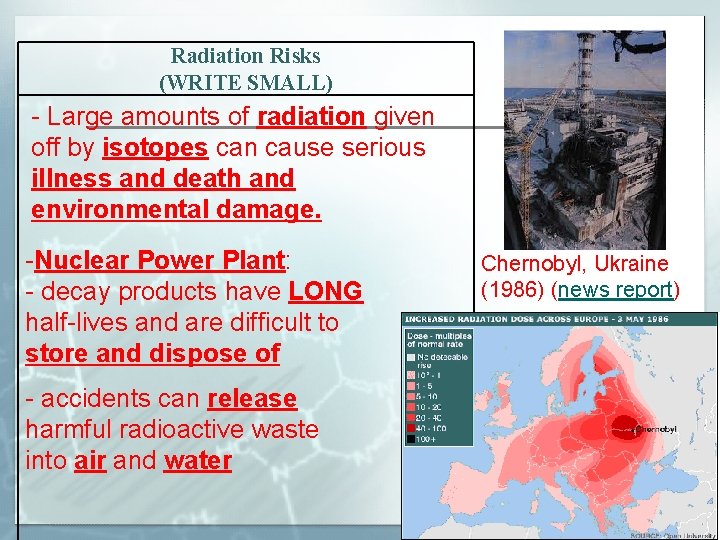
Radiation Risks (WRITE SMALL) - Large amounts of radiation given off by isotopes can cause serious illness and death and environmental damage. -Nuclear Power Plant: - decay products have LONG half-lives and are difficult to store and dispose of - accidents can release harmful radioactive waste into air and water Chernobyl, Ukraine (1986) (news report)