Unit 11 Modern Physics Classical physics particle 1




- Slides: 25

Unit 11 – Modern Physics


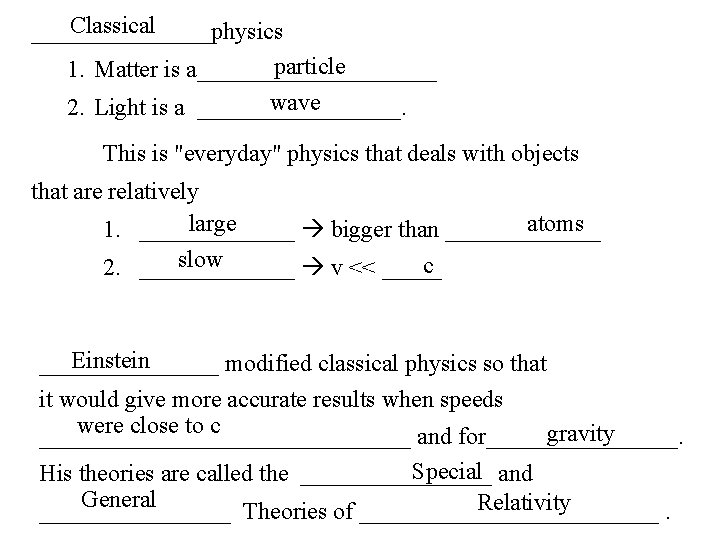
Classical ________physics particle 1. Matter is a__________ wave 2. Light is a _________. This is "everyday" physics that deals with objects that are relatively large atoms 1. _______ bigger than _______ slow c 2. _______ v << _____ Einstein ________ modified classical physics so that it would give more accurate results when speeds were close to c gravity ________________ and for________. Special and His theories are called the ________ General Relativity ________ Theories of _____________.


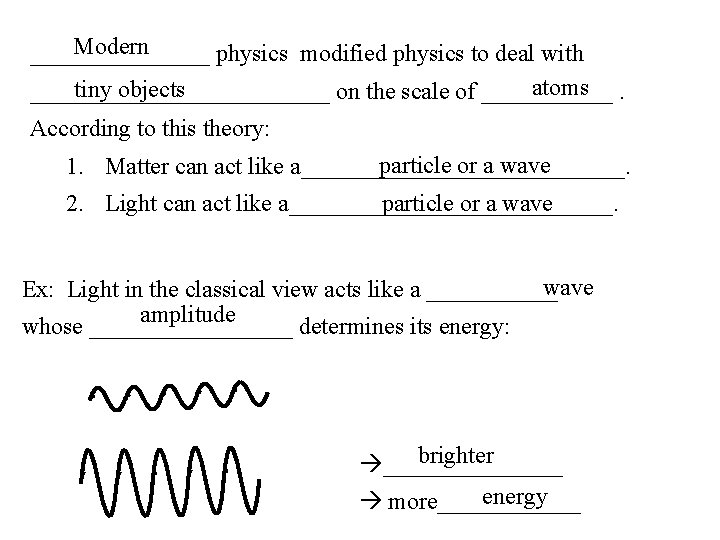
Modern ________ physics modified physics to deal with atoms. tiny objects _____________ on the scale of ______ According to this theory: particle or a wave 1. Matter can act like a______________. particle or a wave 2. Light can act like a______________. wave Ex: Light in the classical view acts like a ______ amplitude whose _________ determines its energy: brighter ________ energy more______

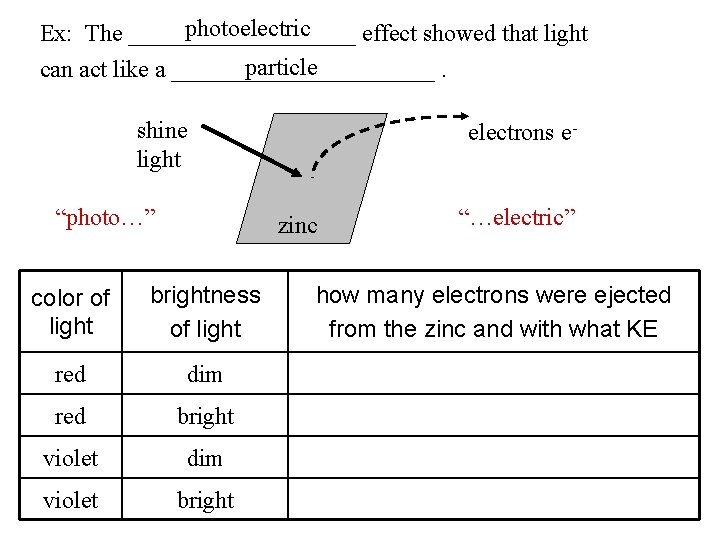
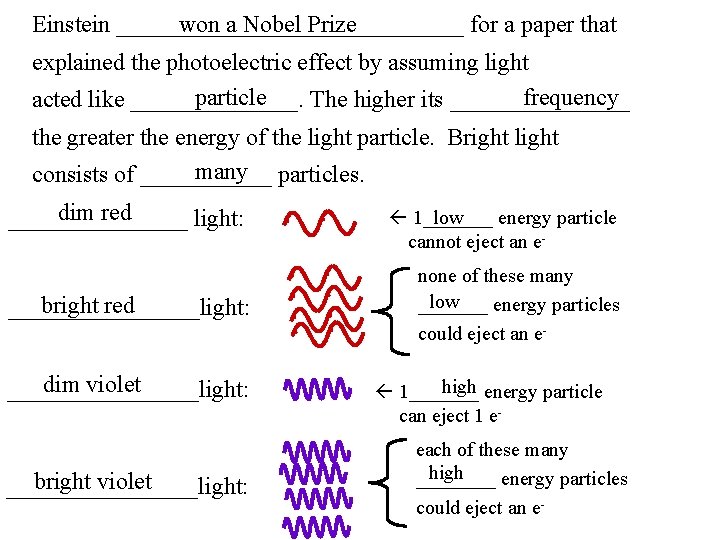
photoelectric Ex: The __________ effect showed that light particle can act like a ___________. shine light “photo…” electrons e- zinc “…electric” color of light brightness of light how many electrons were ejected from the zinc and with what KE red dim no e- red bright no e- violet dim a few e- with lots of KE violet bright lots of e- with lots of KE

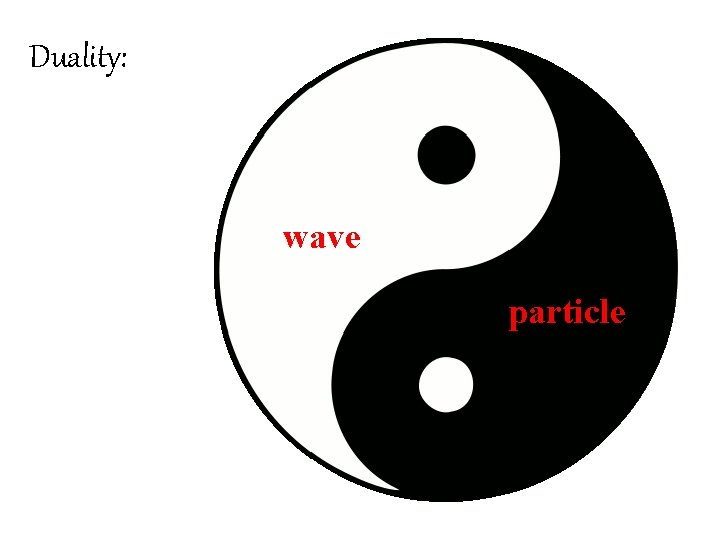
Duality: wave particle

won a Nobel Prize Einstein _______________ for a paper that explained the photoelectric effect by assuming light frequency particle acted like _______. The higher its ________ the greater the energy of the light particle. Bright light many particles. consists of ______ dim red ________ light: bright red ________light: dim violet ________light: bright violet ________light: 1_______ energy particle low cannot eject an enone of these many low _______ energy particles could eject an ehigh energy particle 1_______ can eject 1 eeach of these many high ____ energy particles could eject an e-

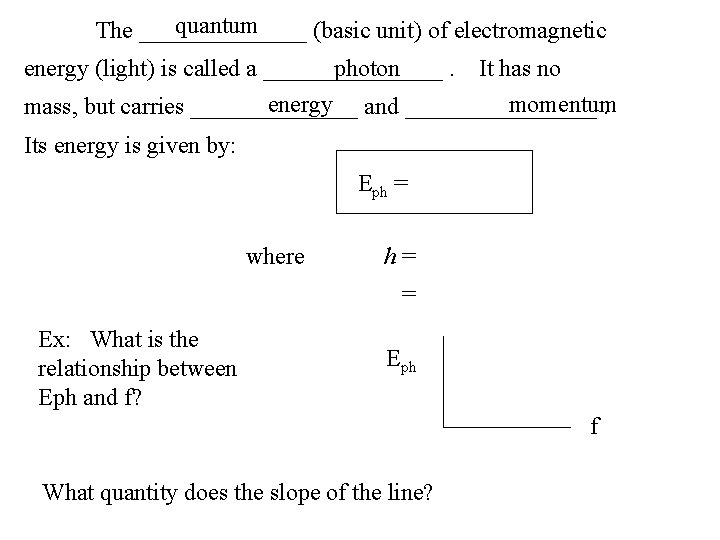
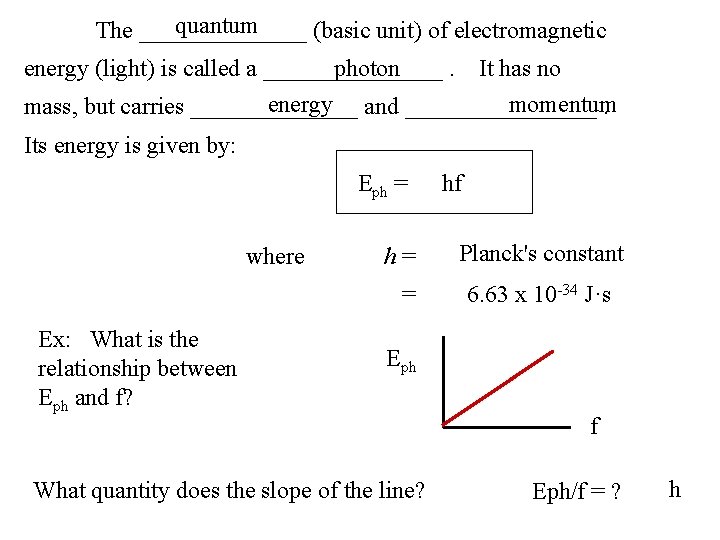
quantum The _______ (basic unit) of electromagnetic energy (light) is called a ________. photon It has no energy and ________ momentum mass, but carries _______. Its energy is given by: Eph = where h= = Ex: What is the relationship between Eph and f? Eph f What quantity does the slope of the line?

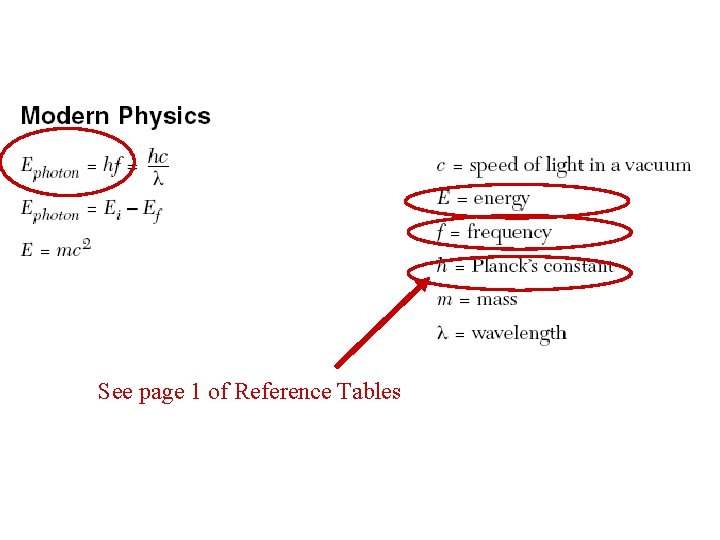
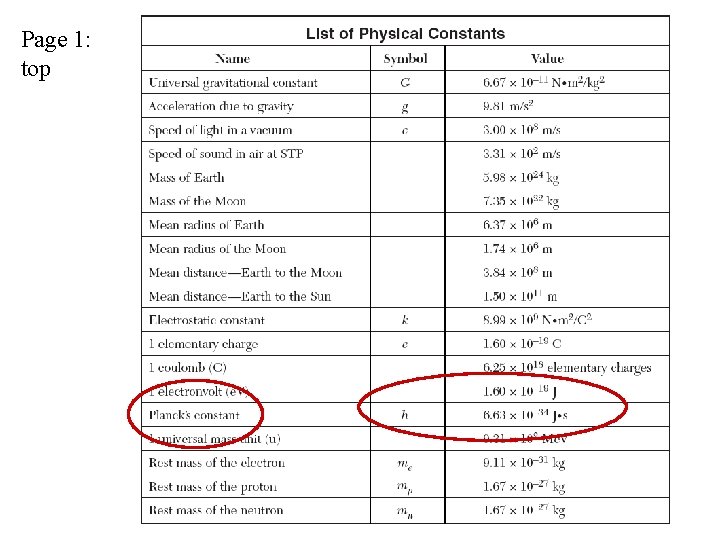
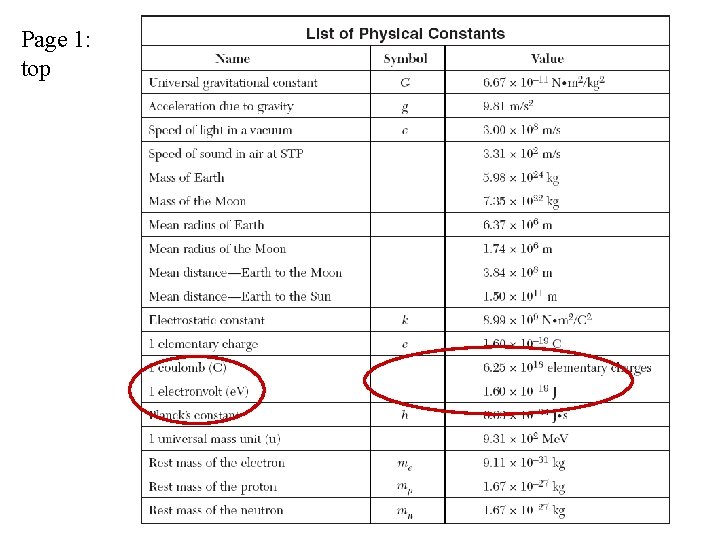
See page 1 of Reference Tables

Page 1: top

quantum The _______ (basic unit) of electromagnetic energy (light) is called a ________. photon It has no energy and ________ momentum mass, but carries _______. Its energy is given by: Eph = where h= = Ex: What is the relationship between Eph and f? hf Planck's constant 6. 63 x 10 -34 J·s Eph What quantity does the slope of the line? f Eph/f = ? h


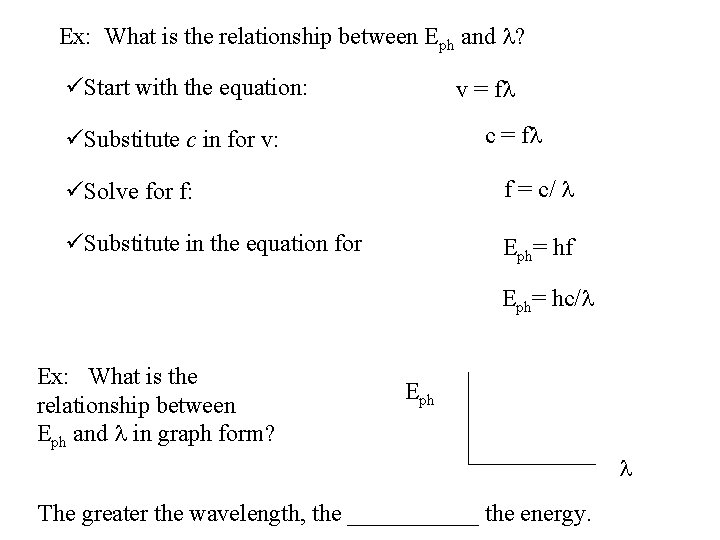
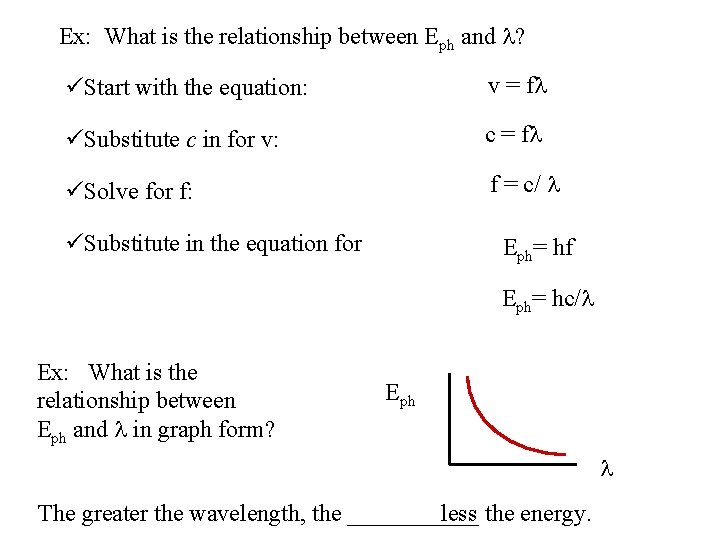
Ex: What is the relationship between Eph and l? üStart with the equation: v = fl c = fl üSubstitute c in for v: üSolve for f: f = c/ l üSubstitute in the equation for Eph= hf Eph= hc/l Ex: What is the relationship between Eph and l in graph form? Eph l The greater the wavelength, the ______ the energy.


Ex: What is the relationship between Eph and l? üStart with the equation: v = fl üSubstitute c in for v: c = fl üSolve for f: f = c/ l üSubstitute in the equation for Eph= hf Eph= hc/l Ex: What is the relationship between Eph and l in graph form? Eph l less the energy. The greater the wavelength, the ______

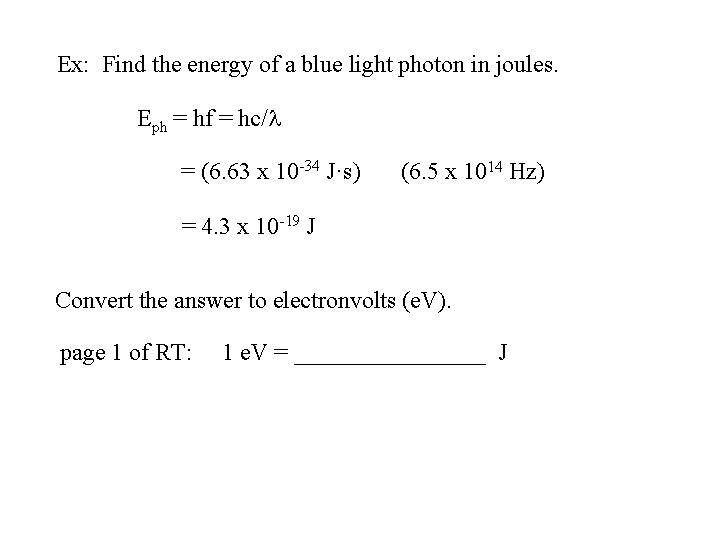
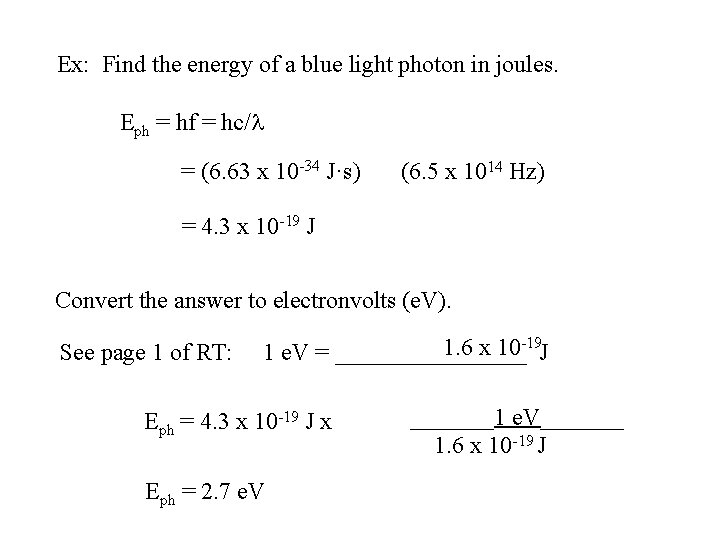
Ex: Find the energy of a blue light photon in joules. Eph = hf = hc/l = (6. 63 x 10 -34 J·s) (? ? ? ) Convert the answer to electronvolts (e. V). page 1 of RT: 1 e. V = ________ J

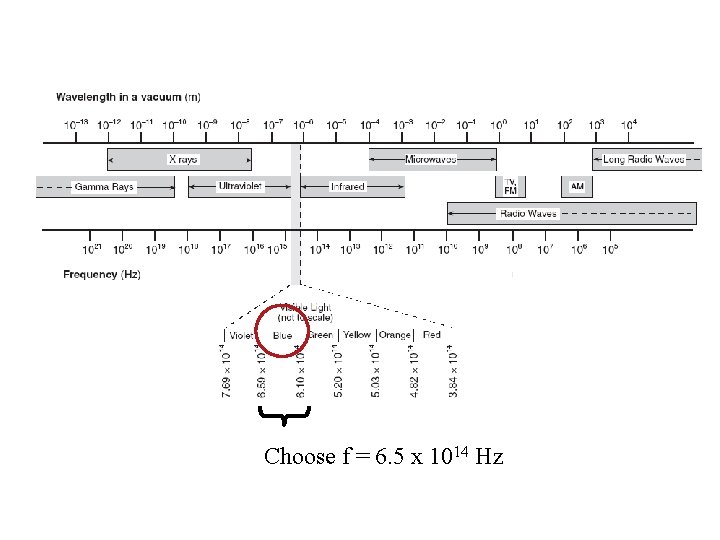
Choose f = 6. 5 x 1014 Hz

Ex: Find the energy of a blue light photon in joules. Eph = hf = hc/l = (6. 63 x 10 -34 J·s) (6. 5 x 1014 Hz) = 4. 3 x 10 -19 J Convert the answer to electronvolts (e. V). page 1 of RT: 1 e. V = ________ J

Page 1: top

Ex: Find the energy of a blue light photon in joules. Eph = hf = hc/l = (6. 63 x 10 -34 J·s) (6. 5 x 1014 Hz) = 4. 3 x 10 -19 J Convert the answer to electronvolts (e. V). See page 1 of RT: 1. 6 x 10 -19 J 1 e. V = ________ Eph = 4. 3 x 10 -19 J x Eph = 2. 7 e. V _______1 e. V_______ 1. 6 x 10 -19 J

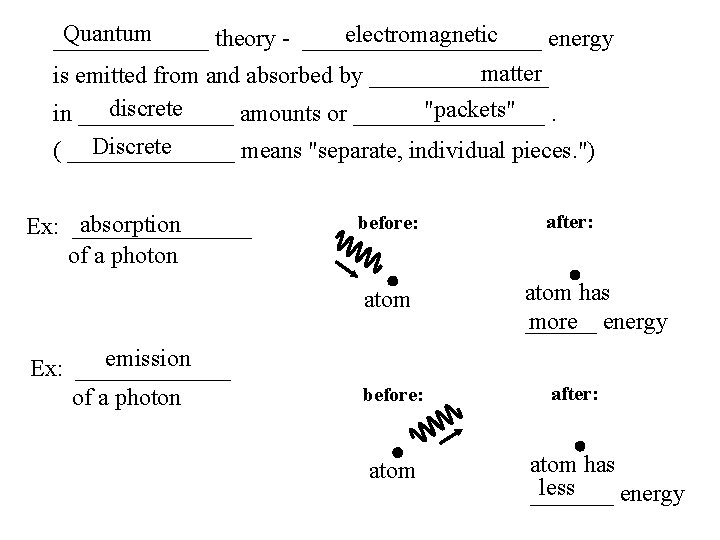
Quantum electromagnetic _______ theory - __________ energy matter is emitted from and absorbed by ________ discrete "packets". in _______ amounts or ________ Discrete ( _______ means "separate, individual pieces. ") absorption Ex: ________ of a photon before: atom emission Ex: _______ of a photon before: atom after: atom has ______ more energy after: atom has less _______ energy

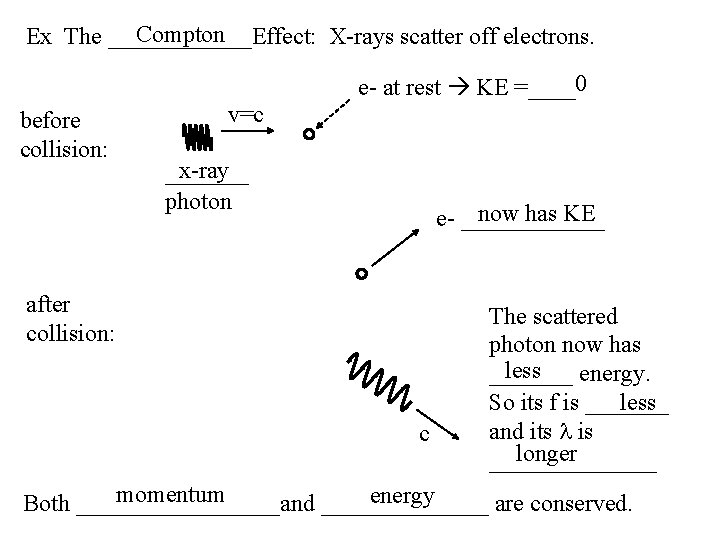
Compton Ex The ______Effect: X-rays scatter off electrons. e- at rest KE =____0 before collision: v=c x-ray _______ photon now has KE e- ______ after collision: c The scattered photon now has less _______ energy. less So its f is _______ and its l is longer _______ momentum energy Both _________and _______ are conserved.

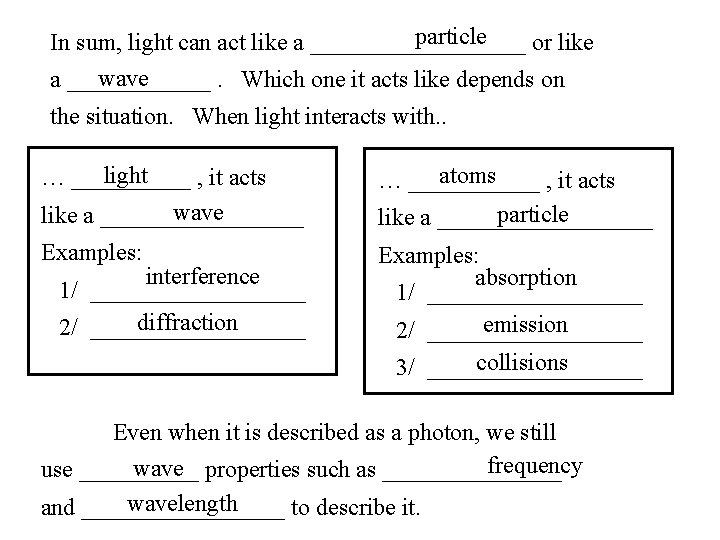
particle In sum, light can act like a _________ or like wave a ______. Which one it acts like depends on the situation. When light interacts with. . light … _____ , it acts wave like a _________ atoms … ______ , it acts particle like a _________ Examples: absorption 1/ _________ emission 2/ _________ interference 1/ _________ diffraction 2/ _________ collisions 3/ _________ Even when it is described as a photon, we still frequency wave properties such as ________ use _____ wavelength and _________ to describe it.

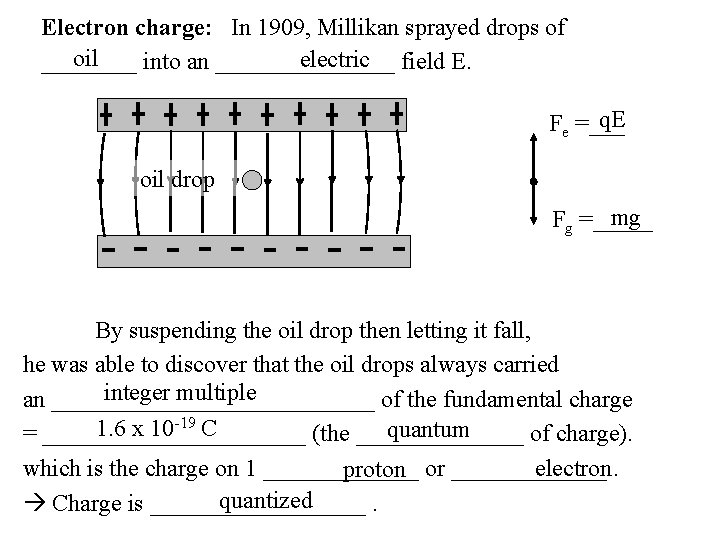
Electron charge: In 1909, Millikan sprayed drops of oil electric field E. ____ into an ________ q. E Fe =___ oil drop mg Fg =_____ By suspending the oil drop then letting it fall, he was able to discover that the oil drops always carried integer multiple an ______________ of the fundamental charge 1. 6 x 10 -19 C quantum = ___________ (the _______ of charge). electron. which is the charge on 1 _______ proton or _______ quantized Charge is _________.