Unit 11 Acids Bases and Solutions Neutralization Reactions

Unit 11: Acids, Bases, and Solutions Neutralization Reactions and Titrations

After today you will be able to… • Explain what a neutralization reaction is • Write the corresponding formulas in neutralization reactions • Calculate an unknown concentration of an acid or base using a titration

Neutralization Reactions A neutralization reaction is a double replacement reaction between an acid and a base to produce water and a salt (an ionic compound).

Examples Predict the products in words and formulas, and balance! hydrogen sulfite sulfurous potassium + water + potassium acid hydroxide sulfite Predict in formulas only and balance. Ca+2 (PO 4)-3 H(OH) 3 Ca(OH)2 + __ 2 H 3(PO 4) __ 1 Ca 3(PO 4)2 6 H 2 O + __ __ 3 1 Ca 3 6 2 (OH) 1 6 63 H 1 6 2 1 (PO 4) 2

In a titration, a solution of known concentration (called the standard solution) is used to determine the concentration of an unknown solution.

Titrations A known quantity of one solution is measured out, and the other solution is added from the buret until the two solutions have neutralized each other. The point at which two solutions have neutralized each other is called the endpoint of the titration.
Titrations An indicator is used to mark the endpoint of the titration. • Phenolphthalein: Is an indicator often used in acidbase titrations. It is colorless in acid and pink in base.

Titration Setup
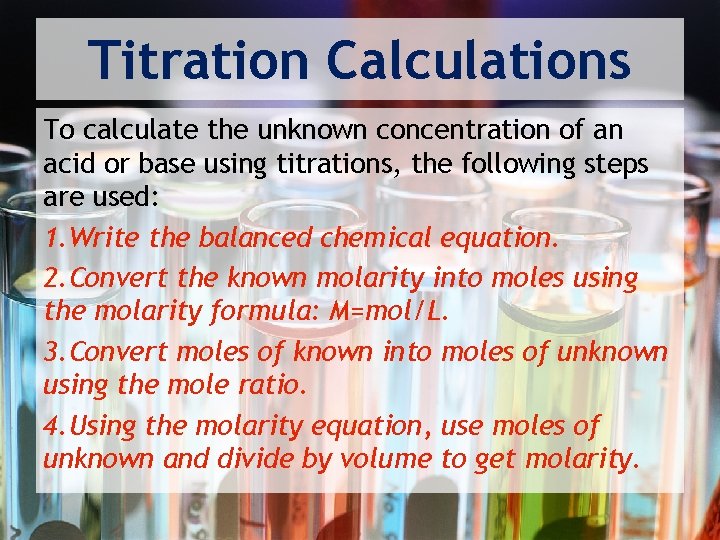
Titration Calculations To calculate the unknown concentration of an acid or base using titrations, the following steps are used: 1. Write the balanced chemical equation. 2. Convert the known molarity into moles using the molarity formula: M=mol/L. 3. Convert moles of known into moles of unknown using the mole ratio. 4. Using the molarity equation, use moles of unknown and divide by volume to get molarity.
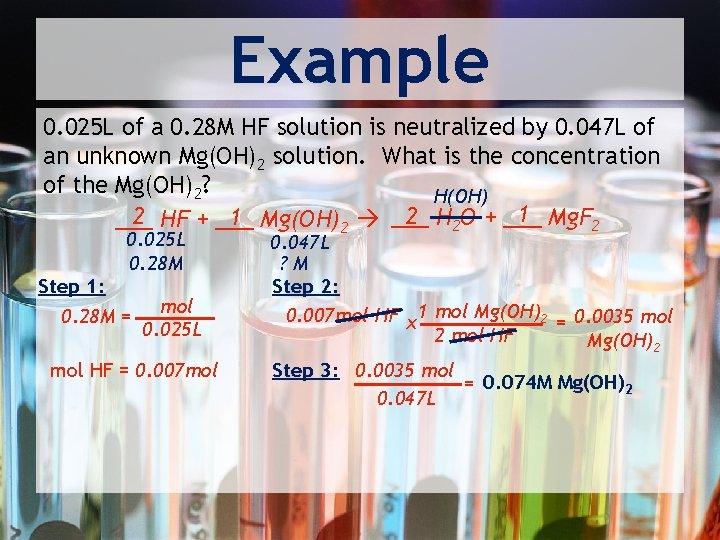
Example 0. 025 L of a 0. 28 M HF solution is neutralized by 0. 047 L of an unknown Mg(OH)2 solution. What is the concentration of the Mg(OH)2? H(OH) 1 Mg. F 2 2 H 2 O + ___ 2 HF + ___ 1 Mg(OH)2 ___ 0. 025 L 0. 28 M Step 1: 0. 28 M = mol 0. 025 L mol HF = 0. 007 mol 0. 047 L ? M Step 2: 0. 007 mol HF x 1 mol Mg(OH)2 = 0. 0035 mol 2 mol HF Mg(OH)2 Step 3: 0. 0035 mol = 0. 074 M Mg(OH)2 0. 047 L
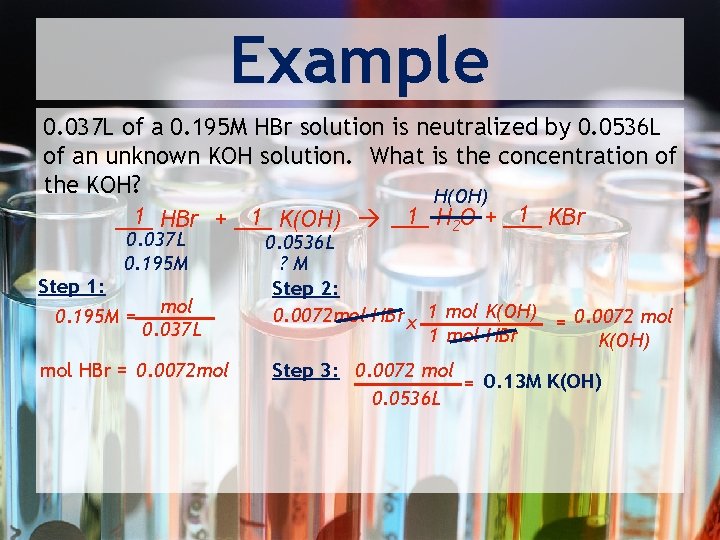
Example 0. 037 L of a 0. 195 M HBr solution is neutralized by 0. 0536 L of an unknown KOH solution. What is the concentration of the KOH? H(OH) 1 KBr 1 H 2 O + ___ 1 HBr + ___ 1 K(OH) ___ 0. 037 L 0. 195 M Step 1: 0. 195 M = mol 0. 037 L mol HBr = 0. 0072 mol 0. 0536 L ? M Step 2: 0. 0072 mol HBr x 1 mol K(OH) = 0. 0072 mol 1 mol HBr K(OH) Step 3: 0. 0072 mol = 0. 13 M K(OH) 0. 0536 L
- Slides: 11