Unit 11 Acids and Bases 11 1 Properties
Unit 11: Acids and Bases � 11. 1 Properties � 11. 2 p. H � 11. 3 Titrations � 11. 4 Definitions

11. 1 Properties � Learning Targets �Describe �Identify the properties of acids and bases. a substance as an acid or base.

11. 1 Properties �Properties of Acids A. Sour taste (Latin: acidus: tart/sour) � Foods that are sour Citrus fruits, vinegar, milk, soda

11. 1 Properties B. Corrosive �Burn/stings C. skin React with metals �Many produce H 2 gas

11. 1 Properties D. Conduct electricity �Acids exist as ions in water �Produce H+1 ions E. Neutralize bases �React with bases to form water and ionic compounds
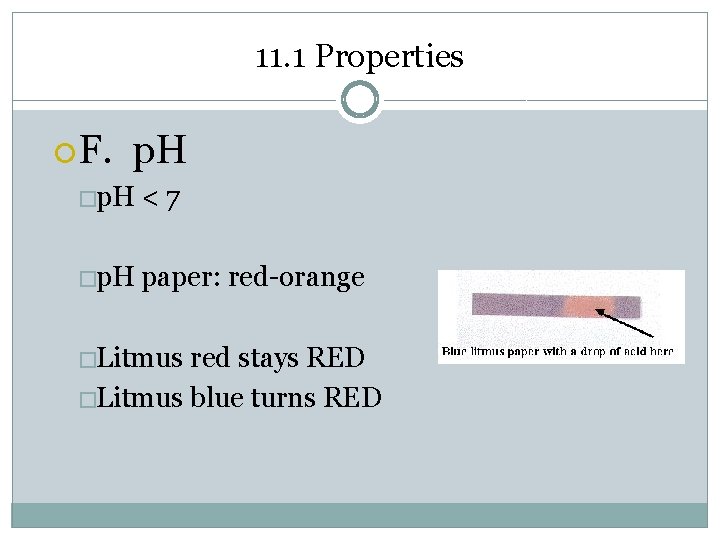
11. 1 Properties F. p. H �p. H <7 �p. H paper: red-orange �Litmus red stays RED �Litmus blue turns RED

11. 1 Properties �Properties of Bases A. Bitter taste �Soap, B. cleaning supplies, toothpaste Soapy/slippery feel

11. 1 Properties C. Nonreactive with metals D. Conducts electricity �Bases exist as ions in water �Produce OH-1 ions E. Neutralize acids �React with acids to form water and ionic compounds
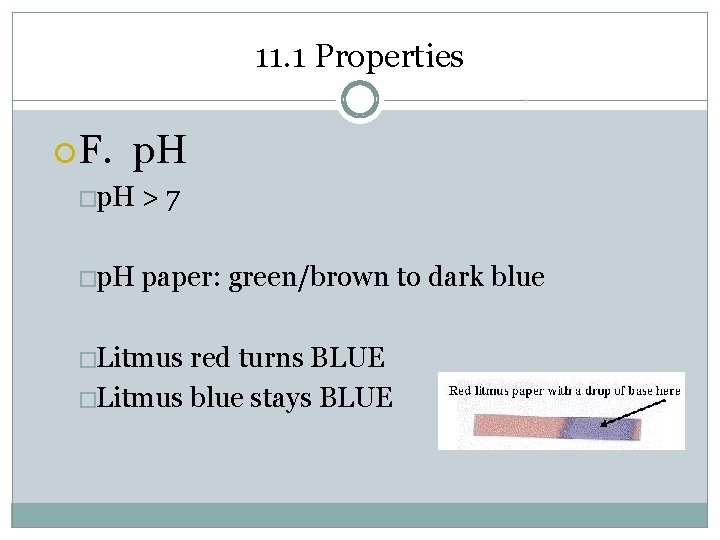
11. 1 Properties F. p. H �p. H >7 �p. H paper: green/brown to dark blue �Litmus red turns BLUE �Litmus blue stays BLUE
11. 1 Properties �Ionic Compound Metal cation and nonmetal anion � aka “salts” A. Product of acid reacting with base � Acid and base “neutralize” each other B. Conduct electricity � Salts exist as ions in water � Ions conduct

11. 2 p. H � Learning Targets � Calculate the p. H, p. OH, [H+], and [OH-] of a solution. � Use indicators to determine the p. H of a solution.
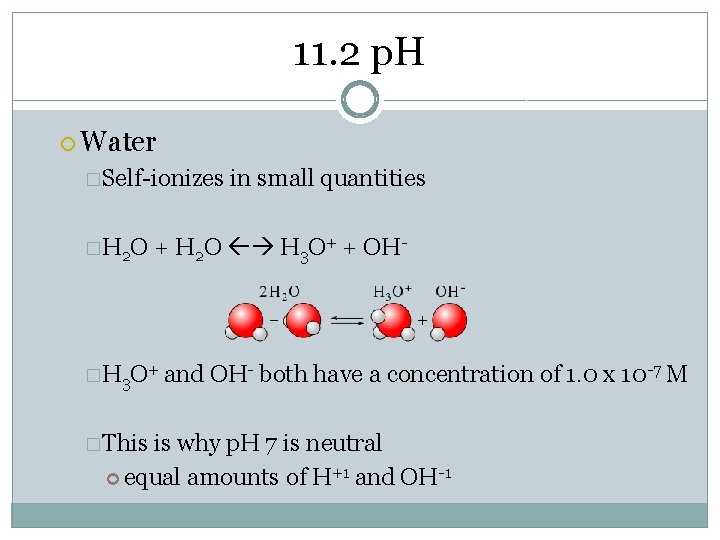
11. 2 p. H Water �Self-ionizes �H 2 O + H 2 O H 3 O+ + OH- �H 3 O+ �This in small quantities and OH- both have a concentration of 1. 0 x 10 -7 M is why p. H 7 is neutral equal amounts of H+1 and OH-1
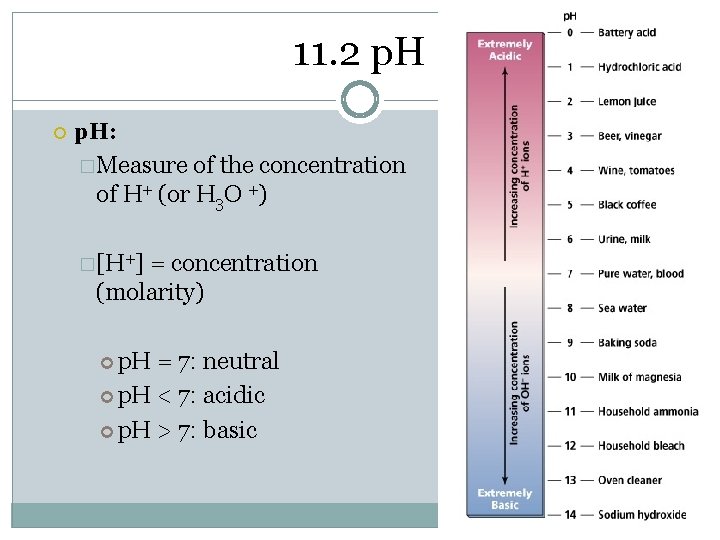
11. 2 p. H: �Measure of the concentration of H+ (or H 3 O +) �[H+] = concentration (molarity) p. H = 7: neutral p. H < 7: acidic p. H > 7: basic
![11. 2 p. H Calculating p. H = -log [H+] must be in Molarity 11. 2 p. H Calculating p. H = -log [H+] must be in Molarity](http://slidetodoc.com/presentation_image_h2/185e382b050528412c2972e9c19c6d76/image-15.jpg)
11. 2 p. H Calculating p. H = -log [H+] must be in Molarity (M) - moles per liter [H+] (-p. H) = 10
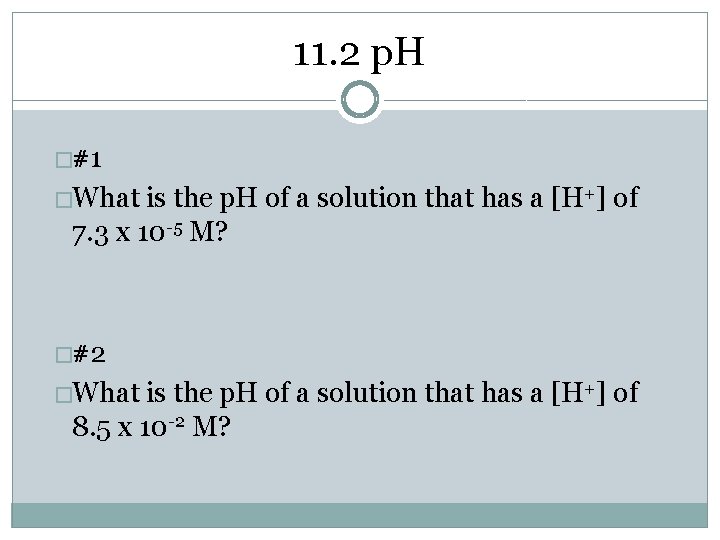
11. 2 p. H �#1 �What is the p. H of a solution that has a [H+] of 7. 3 x 10 -5 M? �#2 �What is the p. H of a solution that has a [H+] of 8. 5 x 10 -2 M?
![11. 2 p. H �#3 �What is the [H+] of a solution that has 11. 2 p. H �#3 �What is the [H+] of a solution that has](http://slidetodoc.com/presentation_image_h2/185e382b050528412c2972e9c19c6d76/image-17.jpg)
11. 2 p. H �#3 �What is the [H+] of a solution that has a p. H of 9. 4? �#4 �What 2. 4? is the [H+] of a solution that has a p. H of
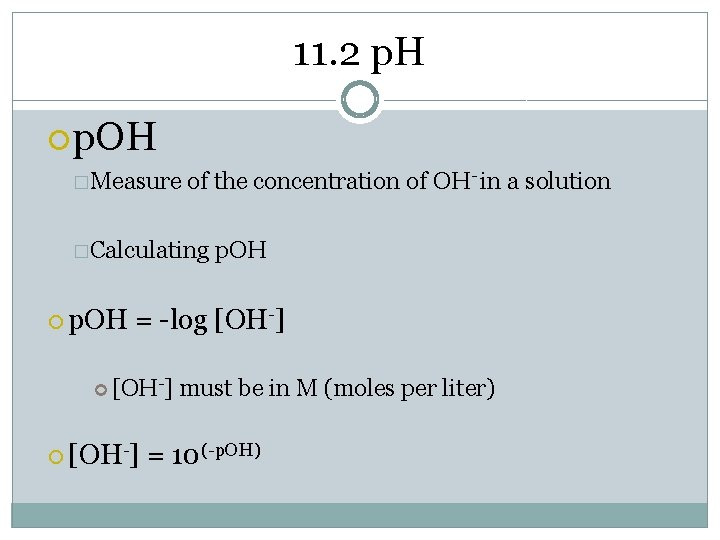
11. 2 p. H p. OH �Measure of the concentration of OH- in a solution �Calculating p. OH = -log [OH-] must be in M (moles per liter) = 10(-p. OH)
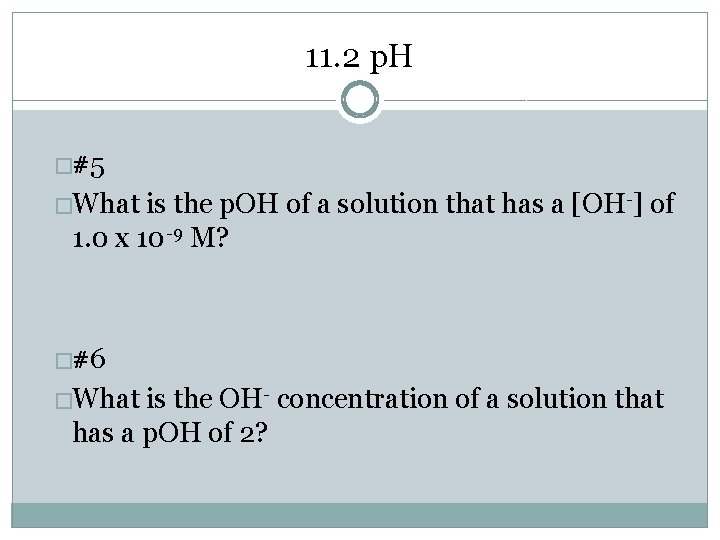
11. 2 p. H �#5 �What is the p. OH of a solution that has a [OH-] of 1. 0 x 10 -9 M? �#6 �What is the OH- concentration of a solution that has a p. OH of 2?
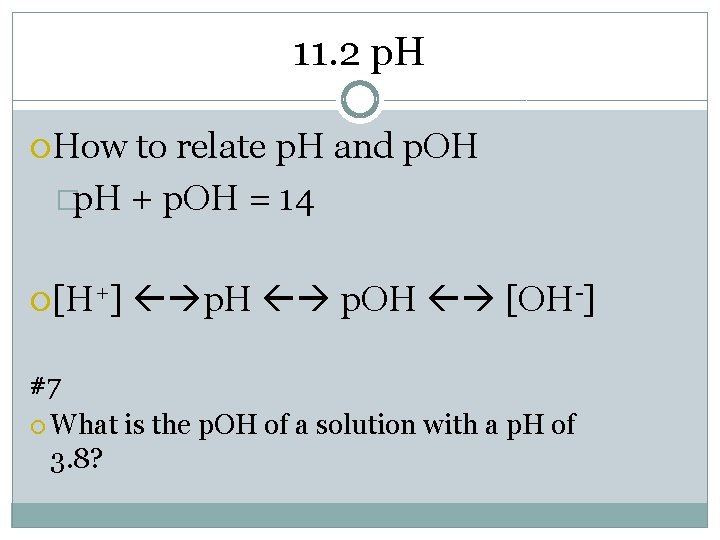
11. 2 p. H How to relate p. H and p. OH �p. H + p. OH = 14 [H+] p. H p. OH [OH-] #7 What is the p. OH of a solution with a p. H of 3. 8?
![11. 2 p. H [H+] p. H p. OH [OH-] #8. What is the 11. 2 p. H [H+] p. H p. OH [OH-] #8. What is the](http://slidetodoc.com/presentation_image_h2/185e382b050528412c2972e9c19c6d76/image-21.jpg)
11. 2 p. H [H+] p. H p. OH [OH-] #8. What is the [OH-] of a solution with a p. H of 4. 2? #9. What is the [OH-] of a solution with a [H+] of 3. 3 x 109?
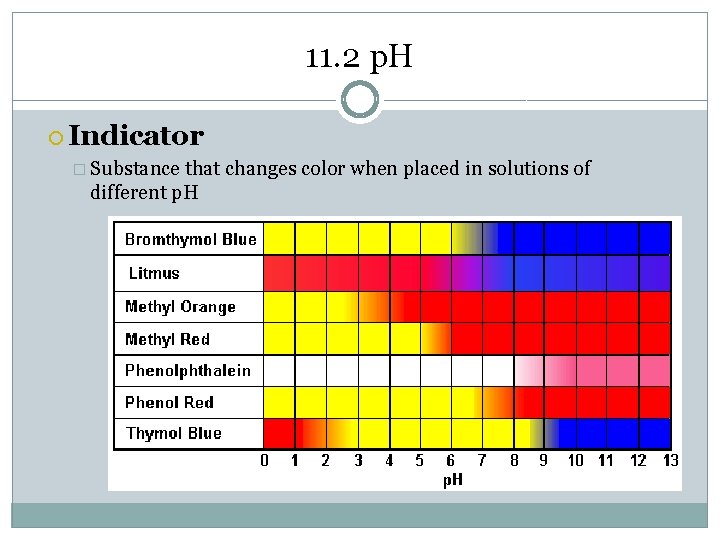
11. 2 p. H Indicator � Substance that changes color when placed in solutions of different p. H
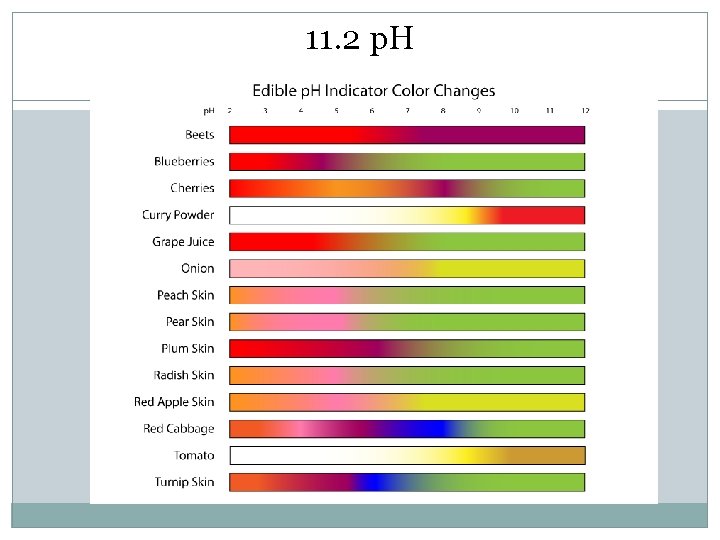
11. 2 p. H

11. 3 Titration � Learning Targets � Predict the products of a neutralization reaction. � Use titration to determine the concentration of an acid or base.
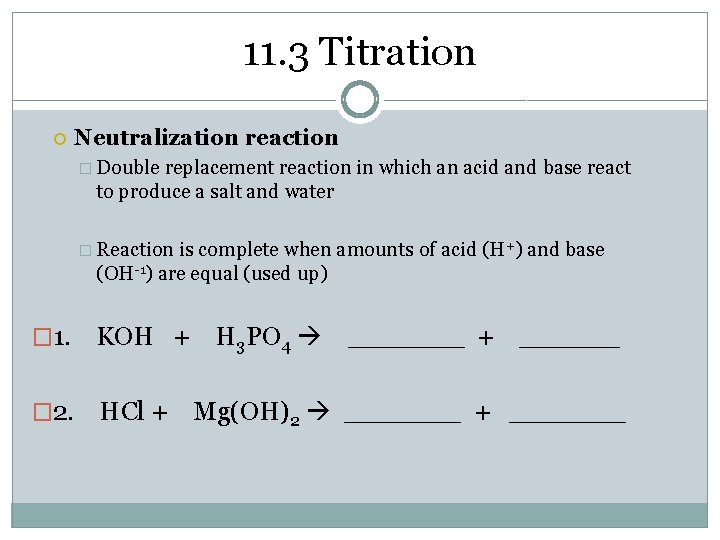
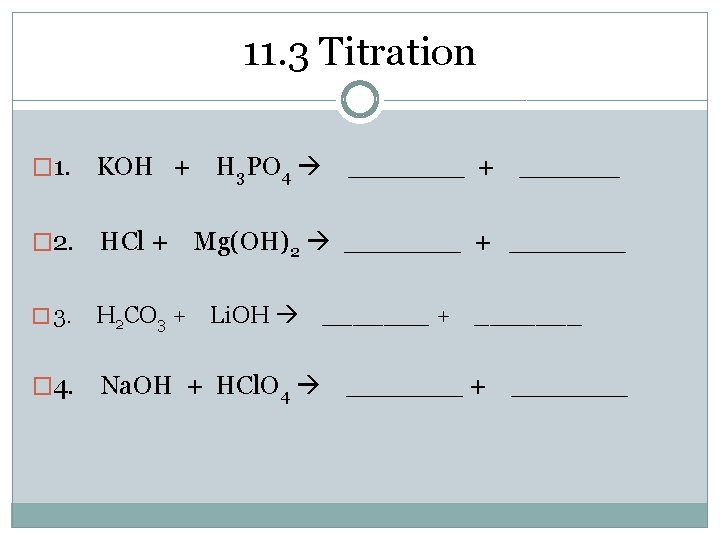
11. 3 Titration Neutralization reaction � Double replacement reaction in which an acid and base react to produce a salt and water � Reaction is complete when amounts of acid (H+) and base (OH-1) are equal (used up) � 1. KOH + H 3 PO 4 _______ + ______ � 2. HCl + Mg(OH)2 _______ + _______

11. 3 Titration �Acid Base Titration Method of determining unknown concentration of an acid or base by neutralizing it with a standard �Standard Solution of a precisely known concentration
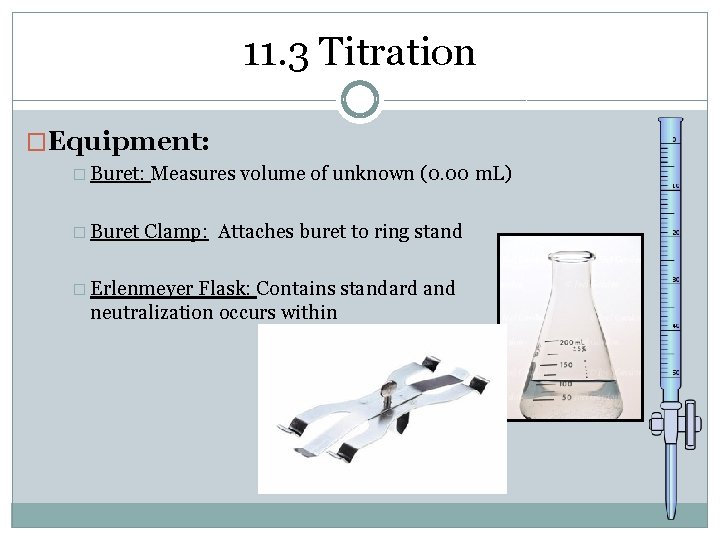
11. 3 Titration �Equipment: � Buret Measures volume of unknown (0. 00 m. L) Clamp: Attaches buret to ring stand � Erlenmeyer Flask: Contains standard and neutralization occurs within
11. 3 Titrations �Sample Titration � Determine concentration of 25. 00 m. L of HCl by neutralizing it with 0. 150 M KOH. � Step 1 The base, the titrant, is placed in the buret. • The titrant is the standard of known concentration. � Step 2 A precise volume of acid is placed in the flask with 2 -3 drops of phenolphthalein indicator. • Since the solution in the flask is acidic, it is colorless.
11. 3 Titration � Step 3 The base is measured exactly and then slowly added while the flask is swirled. The solution turns pink, then colorless again as it mixes. As more KOH is added, more HCl is neutralized. As it takes longer for the pink color to disappear, the KOH is added dropwise. Eventually, all of the acid is used up. Equivalence Point in titration where the H+ and OH-1 ions are equal in quantity

11. 3 Titration � Step 4 A single drop of KOH is added to the colorless solution in the flask. The solution should turn pale pink and stay pink. End Point in titration where indicator changes color permanently The titration is complete. The final volume of titrant is measured.
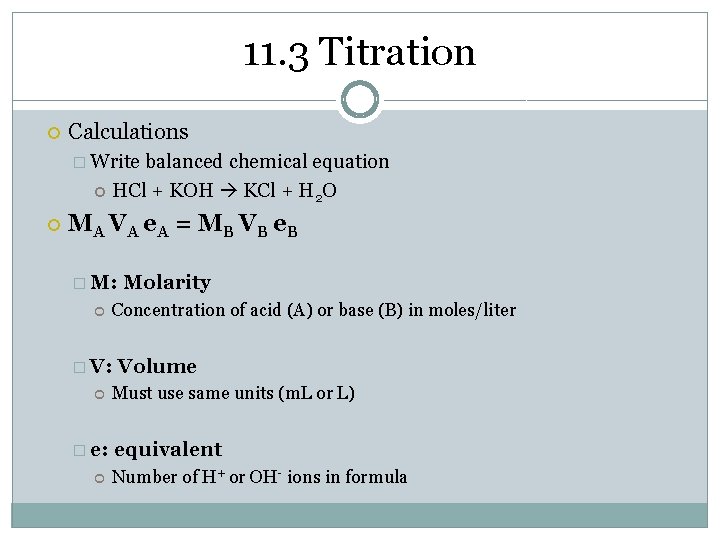
11. 3 Titration Calculations � Write balanced chemical equation HCl + KOH KCl + H 2 O M A VA e A = M B VB e B � M: Concentration of acid (A) or base (B) in moles/liter � V: � e: Molarity Volume Must use same units (m. L or L) equivalent Number of H+ or OH- ions in formula
11. 3 Titration �#1. Determine concentration of 25. 00 m. L of HCl by neutralizing it with 0. 150 M KOH (32. 18 m. L used)

11. 3 Titration #2. In a titration, 22. 17 m. L of 0. 220 M nitric acid HNO 3 was neutralized by 13. 23 m. L of barium hydroxide Ba(OH)2. What is the concentration of the base?
11. 3 Titration �#3. What volume (m. L) of 0. 150 M NH 4 OH solution will neutralize 10. 00 m. L of a 0. 300 M HCl solution?

11. 3 Titration �#4. 13. 88 m. L of a 1. 50 M Na. OH solution neutralizes 27. 76 m. L of a 0. 250 M acid solution. Is the acid HCl, H 2 SO 4, or H 3 PO 4?
11. 3 Titration �#5. A 25. 00 m. L sample of 0. 200 M H 2 C 2 O 4 solution will neutralize 18. 00 m. L of a KOH solution. What mass of KOH was dissolved in the solution?
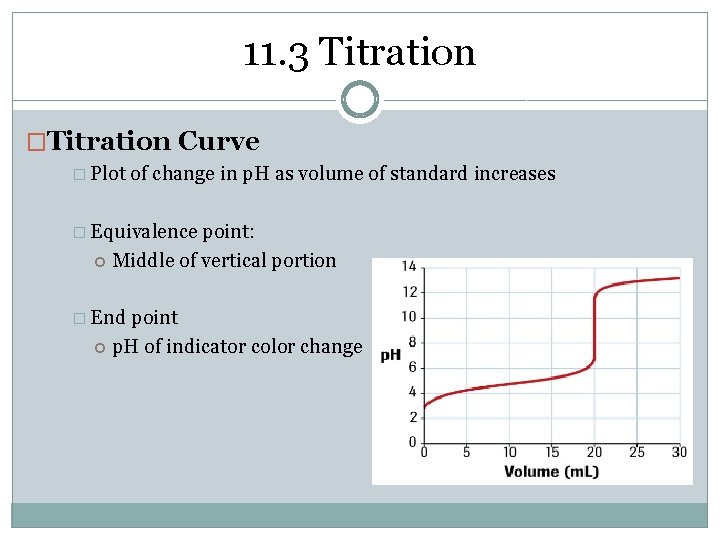
11. 3 Titration �Titration Curve � Plot of change in p. H as volume of standard increases � Equivalence point: Middle of vertical portion � End point p. H of indicator color change

11. 4 Definitions � Learning Targets � Define Arrhenius and Bronsted-Lowry acids and bases. � Identify conjugate acid and conjugate base pairs.
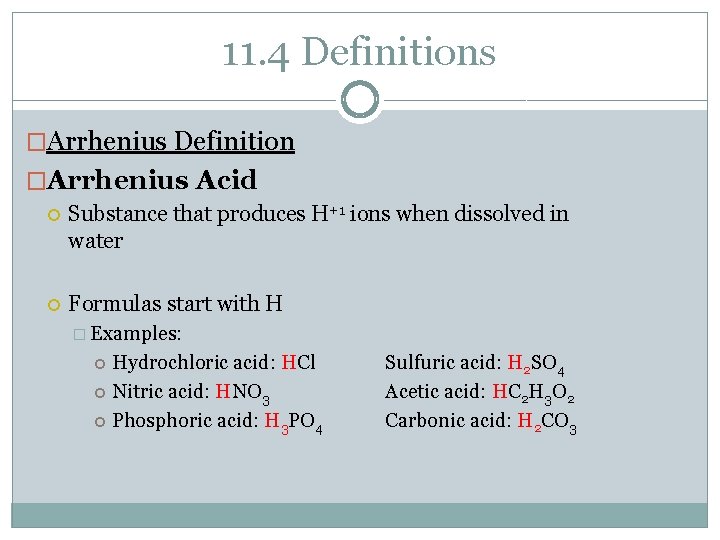
11. 4 Definitions �Arrhenius Definition �Arrhenius Acid Substance that produces H+1 ions when dissolved in water Formulas start with H � Examples: Hydrochloric acid: HCl Nitric acid: HNO 3 Phosphoric acid: H 3 PO 4 Sulfuric acid: H 2 SO 4 Acetic acid: HC 2 H 3 O 2 Carbonic acid: H 2 CO 3

11. 4 Definitions �Arrhenius Base Substance that produces OH-1 ions when dissolved water � Formulas end in OH Examples • • • Sodium hydroxide: Na. OH Potassium hydroxide: KOH Magnesium hydroxide: Mg(OH)2 Calcium hydroxide: Ca(OH)2 Aluminum hydroxide: Al(OH)3 Ammonium hydroxide: NH 4 OH

11. 4 Definitions �Salt Product formed by the neutralization reaction of an acid and base Contains a metal cation (or NH 4+1) Contains an anion �Amphoteric � Substance that can act as both acid or base H 2 O (HOH)

11. 4 Definitions �Dissociation � Acid or base breaks apart into ions when dissolved in water Also called ionization � HCl (aq) � H 3 PO 4 (aq) � Mg(OH)2 (aq) � H 2 SO 4 (aq)

11. 4 Definitions �Strength � Measure of how much an acid or base ionizes when dissolved in water �Strong � Substance completely ionizes/dissociates HCl H+ + Cl- �Weak � Substance does not all dissociate/ionize • H 2 CO 3 2 H+ + CO 3 -2
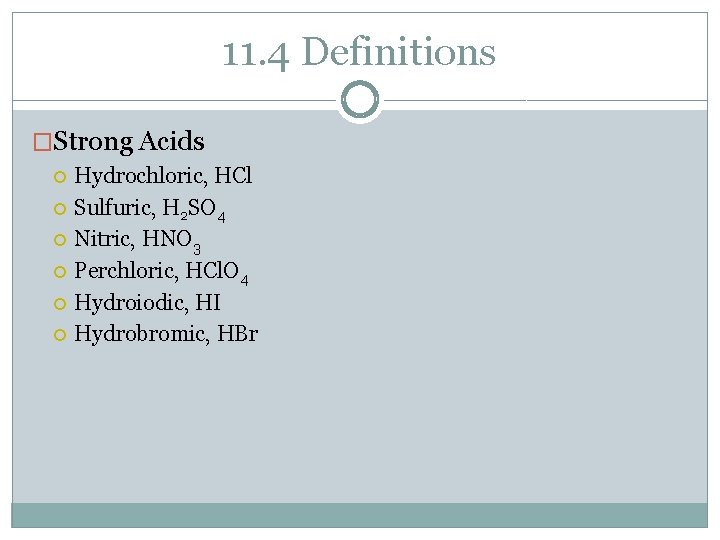
11. 4 Definitions �Strong Acids Hydrochloric, HCl Sulfuric, H 2 SO 4 Nitric, HNO 3 Perchloric, HCl. O 4 Hydroiodic, HI Hydrobromic, HBr

11. 4 Definitions �Strong Bases (Group 1 and 2 hydroxides) Na. OH KOH Li. OH Ba(OH)2 Ca(OH)2 Mg(OH)2 NH 4 OH

11. 4 Definitions �Strength vs Concentration Strength: � How much of it ionizes when dissolved in water Concentration: � How much is dissolved in water (may or may not be ionized)
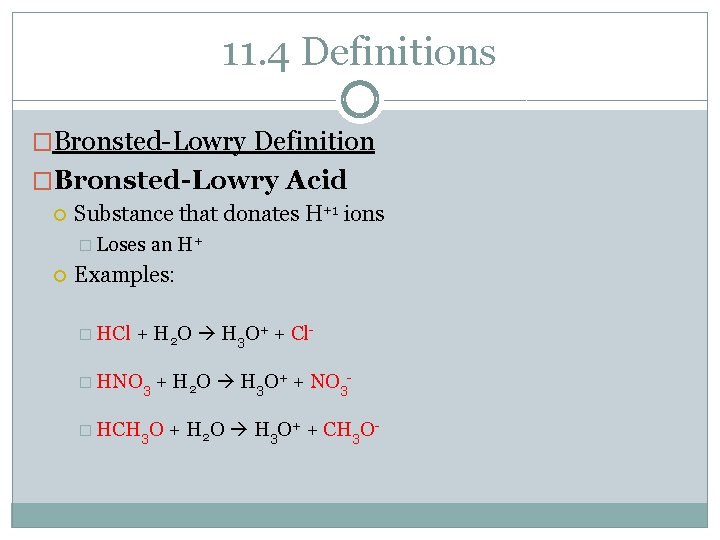
11. 4 Definitions �Bronsted-Lowry Definition �Bronsted-Lowry Acid Substance that donates H+1 ions � Loses an H+ Examples: � HCl + H 2 O H 3 O+ + Cl- � HNO 3 + H 2 O H 3 O+ + NO 3 - � HCH 3 O + H 2 O H 3 O+ + CH 3 O-
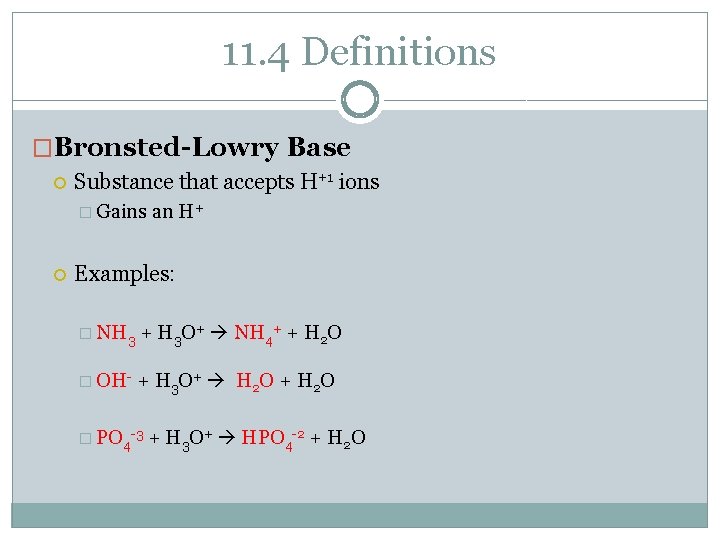
11. 4 Definitions �Bronsted-Lowry Base Substance that accepts H+1 ions � Gains an H+ Examples: � NH 3 + H 3 O+ NH 4+ + H 2 O � OH- + H 3 O+ H 2 O + H 2 O � PO 4 -3 + H 3 O+ HPO 4 -2 + H 2 O
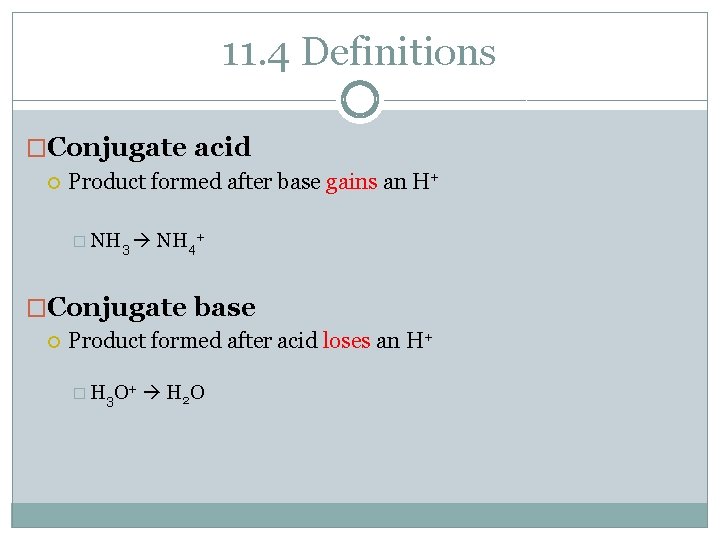
11. 4 Definitions �Conjugate acid Product formed after base gains an H+ � NH 3 NH 4+ �Conjugate base Product formed after acid loses an H+ � H 3 O+ H 2 O

11. 4 Definitions �What is the conjugate acid of A. Br -1 B. CO 3 -2 C. NH 3

11. 4 Definitions �What is the conjugate base of D. H 3 PO 4 E. HCO 3 -1 F. NH 3
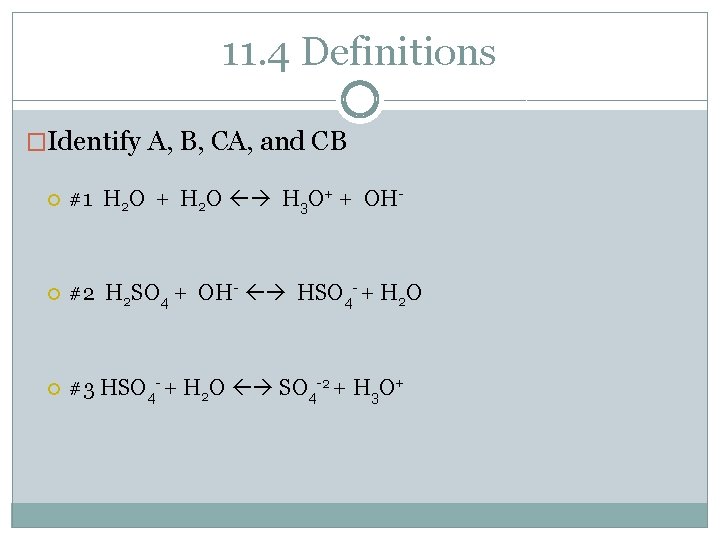
11. 4 Definitions �Identify A, B, CA, and CB #1 H 2 O + H 2 O H 3 O+ + OH- #2 H 2 SO 4 + OH- HSO 4 - + H 2 O #3 HSO 4 - + H 2 O SO 4 -2 + H 3 O+
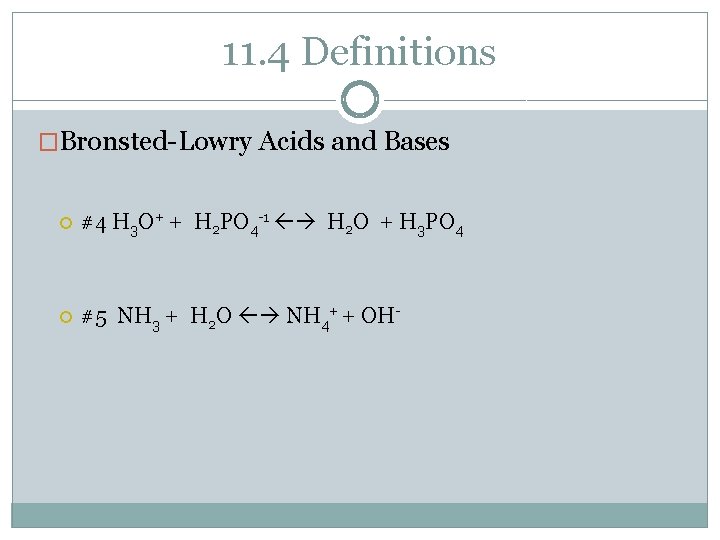
11. 4 Definitions �Bronsted-Lowry Acids and Bases #4 H 3 O+ + H 2 PO 4 -1 H 2 O + H 3 PO 4 #5 NH 3 + H 2 O NH 4+ + OH-
11. 5 Neutralization and Titration � Method of determining the unknown concentration of an acid or base by neutralizing it with a standard Standard � Solution of known concentration used to determine the concentration of an unknown
11. 5 Neutralization and Titration An indicator (color change) is used to tell when the unknown has been neutralized Equivalence point Volume needed for the acid and base to completely neutralize one another
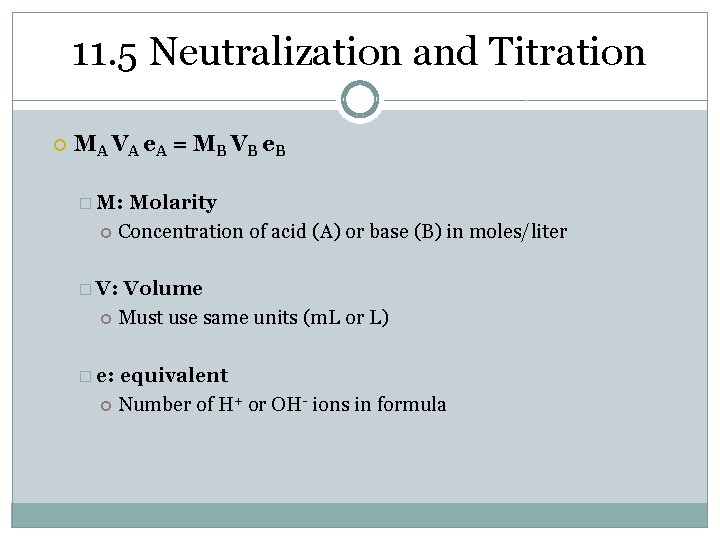
11. 5 Neutralization and Titration M A VA e A = M B VB e B � M: Molarity Concentration of acid (A) or base (B) in moles/liter � V: Volume Must use same units (m. L or L) � e: equivalent Number of H+ or OH- ions in formula
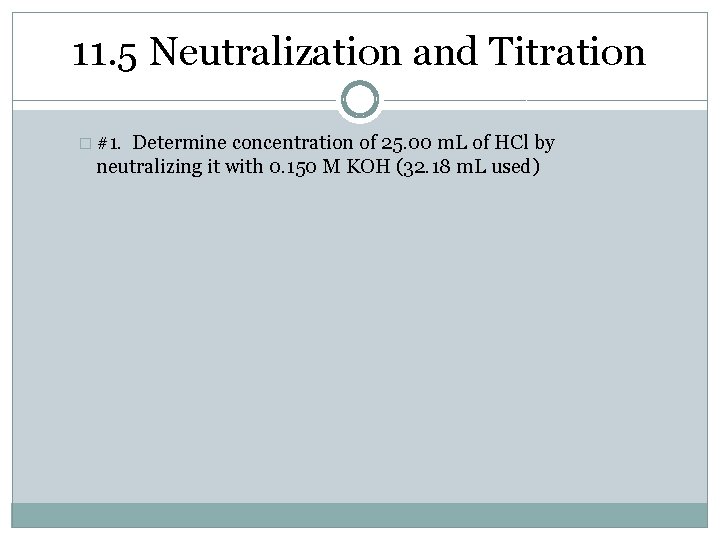
11. 5 Neutralization and Titration � #1. Determine concentration of 25. 00 m. L of HCl by neutralizing it with 0. 150 M KOH (32. 18 m. L used)
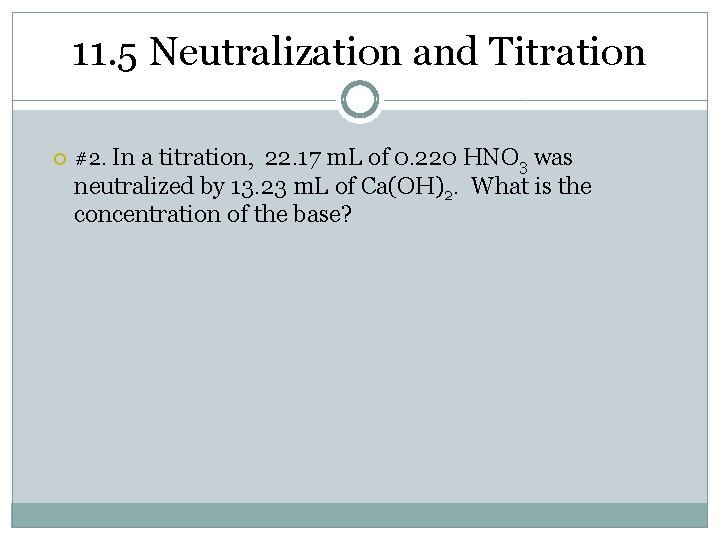
11. 5 Neutralization and Titration #2. In a titration, 22. 17 m. L of 0. 220 HNO 3 was neutralized by 13. 23 m. L of Ca(OH)2. What is the concentration of the base?
- Slides: 63