Unit 10 Summary checkpoint 10 1 Acids alkalis

















- Slides: 20

Unit 10 Summary check-point

10. 1 Acids & alkalis in daily life p. 57 sour (a) but alkalis 1 Acids taste ______ bitter (b). taste ______ 2 The colour of red cabbage extract red in acids & ____ green (b) in is ______ (a) alkalis.

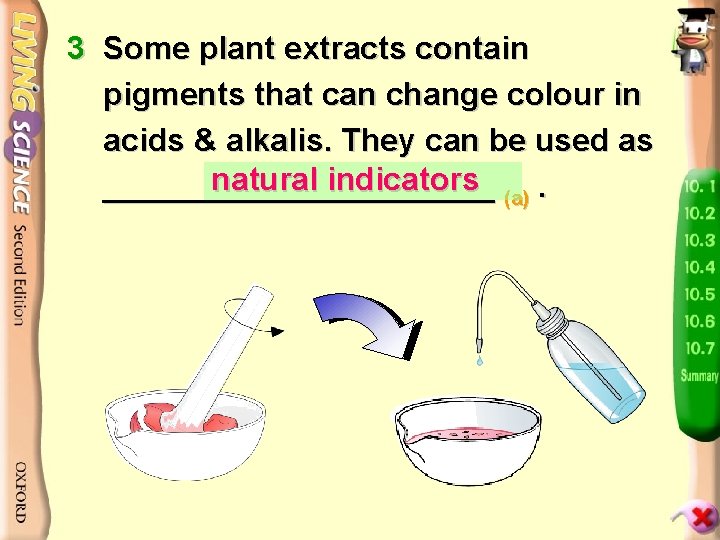
3 Some plant extracts contain pigments that can change colour in acids & alkalis. They can be used as natural indicators (a). ___________

10. 2 Laboratory acids & alkalis 4 The commonly used laboratory acids (a) include hydrochloric _____ acid, nitric acid and sulphuric acid. The commonly used laboratory alkalis (b) include sodium _____ hydroxide & ammonia solution.

5 We should take the necessary safety precautions when __________ (a) handling acids & alkalis. We must use them carefully. 6 Common indicators used in the litmus (a) & laboratory are _____ universal indicator (b). _________ p. 58

red (a) in acids. 7 Blue litmus turns ______ blue (b) in alkalis. Red litmus turns ______ 8 Universal indicator changes to red blue _____ in acids & ______ in (a) (b) alkalis with a series of colours in between. It is green in colour neutral in _____ substances. (c)

p. H scale (a) tells the degree of 9 The _____ acidity & alkalinity of substances. 0 (b) to The p. H value ranges from_____ 14 ____ (c). acidic > The p. H value of _____ (d) substances is below 7. neutral > The p. H value of _____ (e) substances is 7. alkaline > The p. H value of _____ (f) substances is above 7.

10. 3 Importance of keeping the right p. H 10 It is important for the different parts of proper our body to maintain their ____ (a) p. H levels so that our body can function properly. For plants, the availability of nutrients depend on the soil acidity or alkalinity of the ______ (b). 11 Many daily used products like cosmetics & personal care products p. H values show their ______ (a) on their packs.

10. 4 The corrosive effect of acids hydrogen gas 12 We can test for __ ______ (a) by placing a burning splint at the mouth of the test-tube full of the gas. It gives out a ‘pop’ sound. 13 Dilute acids react with some metals hydrogen (a). The to give _______ corrosive acids are _____ to these (b) metals.

14 Dilute acids also react with building calcium carbonate materials made of ________ carbon dioxide (a) to give ________ (b). That (a) (b) is why acids corrode our buildings & statues. sandstone marble limestone

10. 5 Acid rain —— the invisible threat 15 The average p. H value of rainwater 5. 6 is about ______ (a) because carbon dioxide ________ (b) in the air can dissolve in it. The p. H of acid rain is 5. 6 below ______ (c) 。

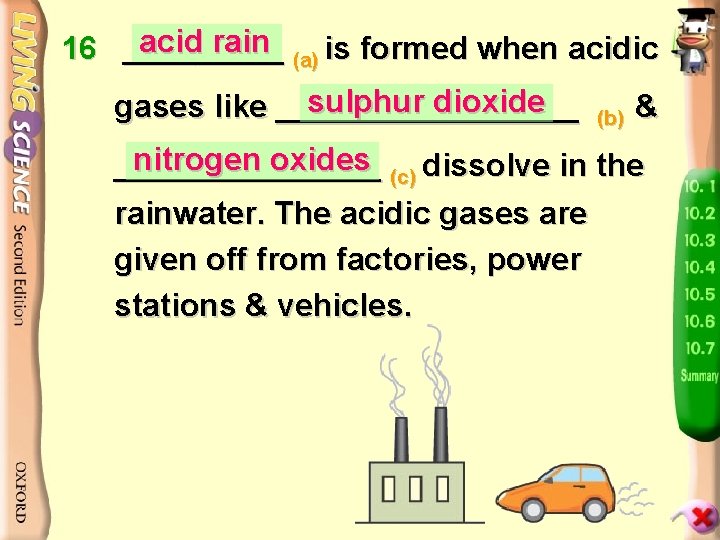
acid rain 16 _____ (a) is formed when acidic sulphur dioxide gases like _________ (b) & nitrogen oxides (c) dissolve in the ________ rainwater. The acidic gases are given off from factories, power stations & vehicles.

p. 59 17 The harmful effect of acid rain is corrodes slow but destructive. It _____ (a) our buildings & statues. It can also destroy life ______ (b) in our rivers, lakes growth & the sea. It can affect the _______ of plants _____ by turning the soil (c) acidic & toxic. 18 We can help reduce air energy pollution by using ____ (a) more efficiently.

10. 6 Everyday uses of acids & alkalis preserve (a) 19 Acids can be used to _____ food because it can stop the growth of micro-organisms. Vinegar (a) contains acetic acid 20 _____ and is commonly used for food preservation.

21 Fruits like apple, banana, pear and peach turn brown when they are cut and left in the air for a period of time. Acids can prevent fruits from browning 。 ______ (a) 22 Acids & alkalis are commonly used cleansing agents as __________ (a) . For example, hydrochloric acid is used in toilet cleansers, caustic soda in oven cleansers and ammonia solution as window cleansers.

neutralize 23 Acids & alkalis _______ (a) each other in a chemical reaction neutralization (b). In called _______ salt (c) & ______ water (d) this reaction, _____ are formed.

24 We can make use of neutralization to solve many daily problems. Examples are: antacid > Use of ______ (a) to treat stomach-ache > Use of acids or alkalis to treat wasp, bee, mosquito & ant stings > Neutralization of acidic & alkaline industrial wastes before disposal _____ (b)

10. 7 Safety matters related to the use of acids & alkalis 25 Acids & alkalis should be handled carefully because they are corrosive ________ (a).

Concentrated (a) acid is a solution 26 ______ that contains a high proportion of Concentrated acid in water. _______ (b) alkali is a solution that contains a high proportion of alkali in water. corrosive They are much more ______ c) than dilute acids & alkalis.

27 Concentrated acids and alkalis dilution give out heat on _____ (a). We must dilute concentrated acids & alkalis by adding them to water, but not by adding water to them. We must wear protective clothes, safety goggles & gloves when doing so. concentrated acid / alkali water