UNIT 10 STATIC ELECTRICITY Chapter 18 TYPES OF

















- Slides: 36

UNIT 10 – STATIC ELECTRICITY Chapter 18

TYPES OF ELECTRIC CHARGE Static (Unit 10) Charge at rest Collection of charge static electricity Dynamic (Unit 11) Charge in motion : current Such as in electrical circuits

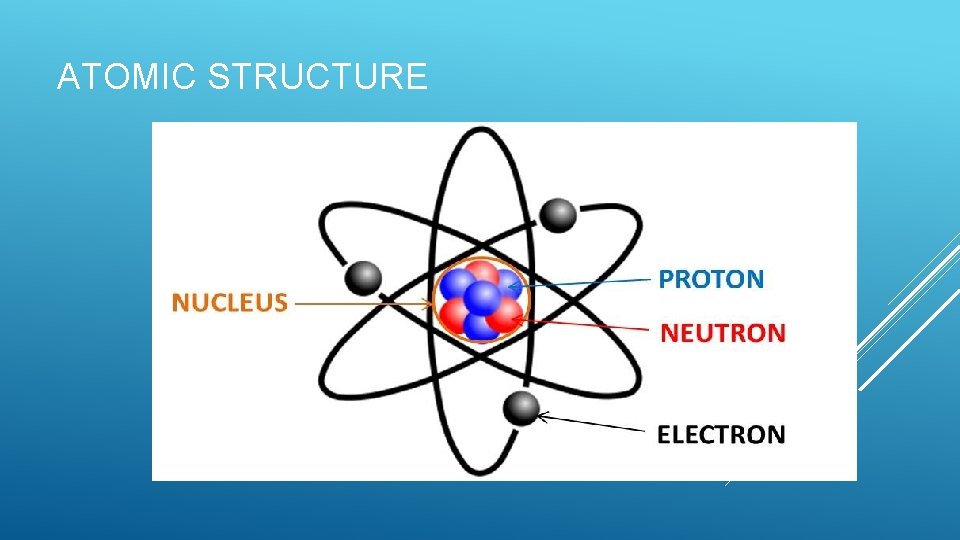
ATOMIC STRUCTURE

ATOMIC STRUCTURE Proton: Positive charge object found in the nucleus Neutron: Neutral object found in the nucleus The nucleus will also have a positive charge since it consists of protons and neutrons Both the protons and the neutrons are stuck in the nucleus. They cannot move! Electron: Negatively charged object found orbiting the nucleus of an atom Can move from object to object or through a material.

THE CHARGE OF AN ATOM If an atom has an equal number of protons and electrons it is considered electrically neutral. Ions (charged atoms) contain a different number of protons and electrons Fewer electrons – positively charged More electrons – negatively charged

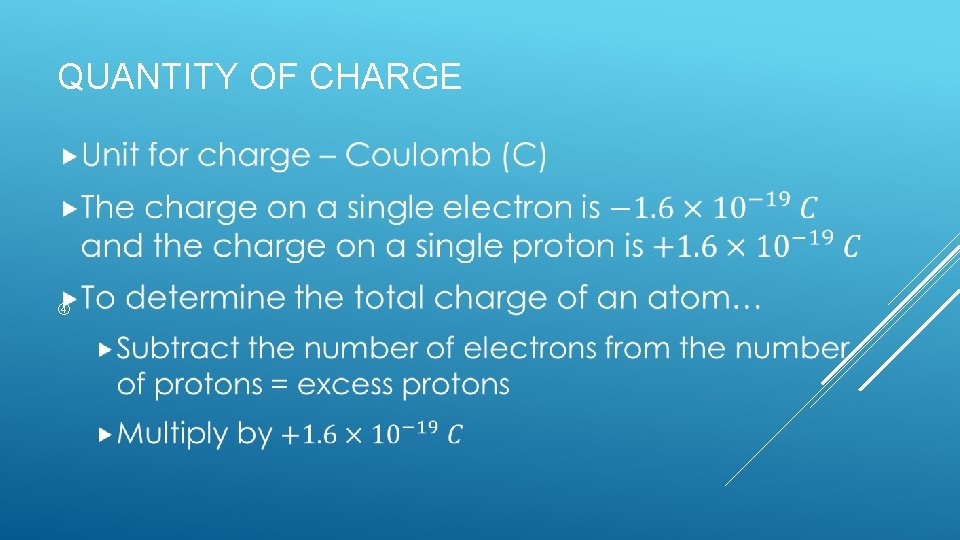
QUANTITY OF CHARGE

EXAMPLE #1

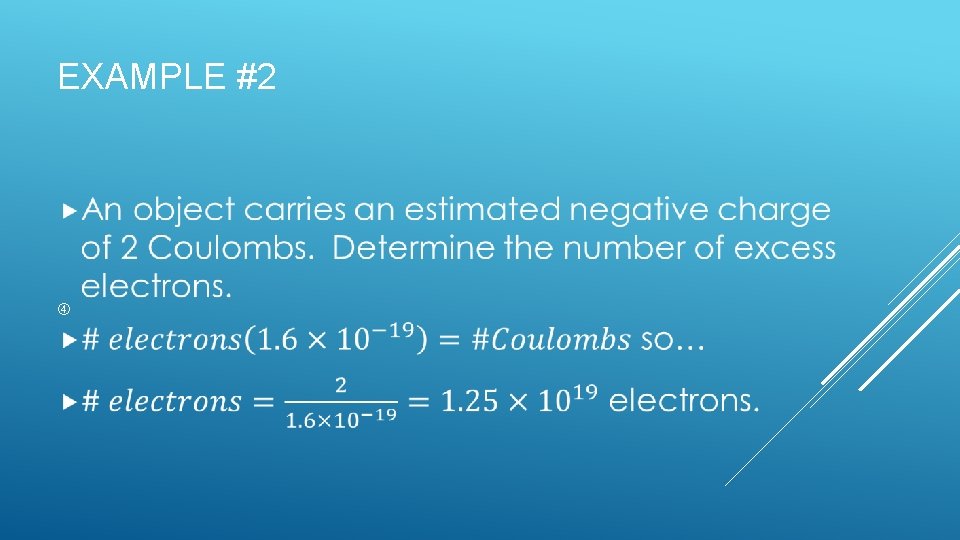
EXAMPLE #2

CHARGE INTERACTIONS Charged objects exert an electric force upon other objects. Non-contact force (like gravity) Rule #1: As Paula Abdul knows, OPPOSITES ATTRACT! + attracts – AND – attracts + Rule #2: Neutral objects are attracted to BOTH positive and negative objects + attracts N AND - attracts N Rule #3: LIKE OBJECTS REPEL + repels + AND - repels -

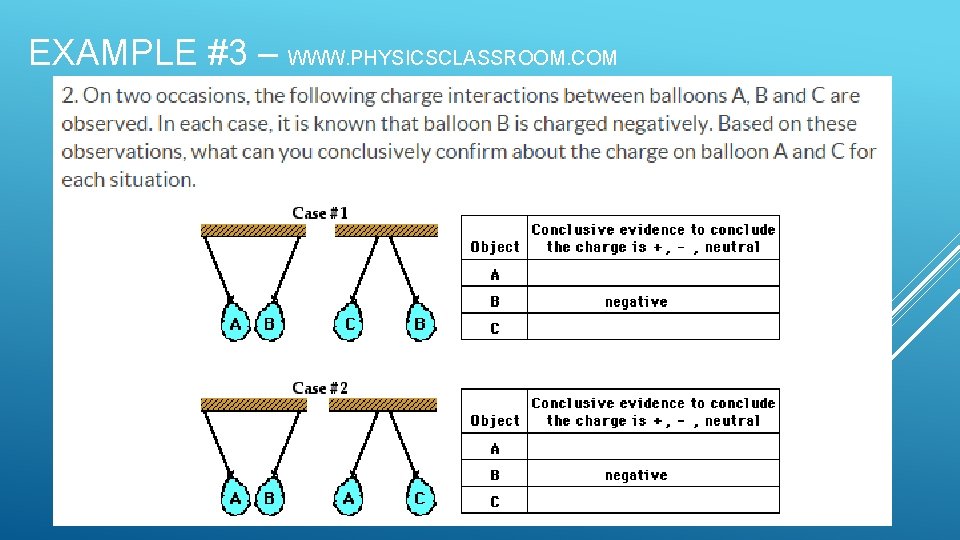
EXAMPLE #3 – WWW. PHYSICSCLASSROOM. COM

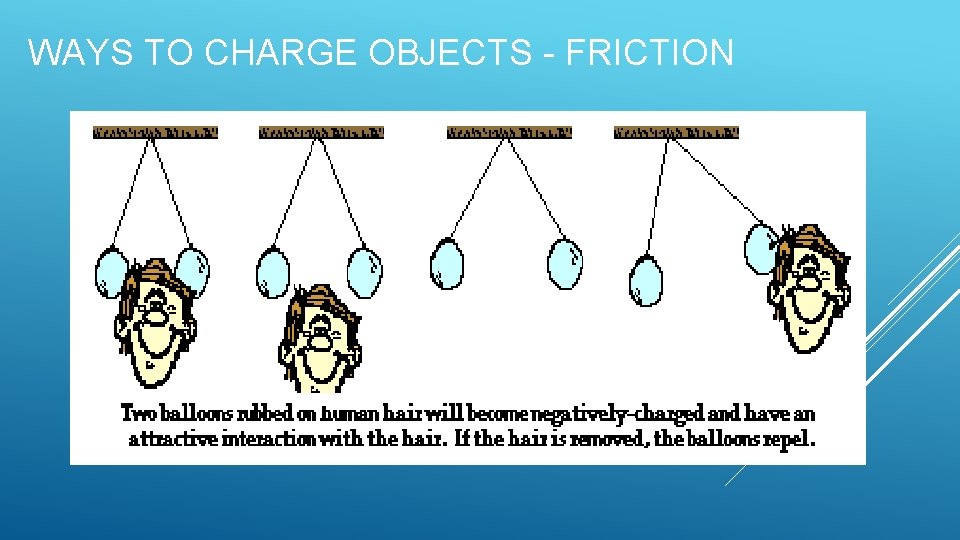
WAYS TO CHARGE OBJECTS - FRICTION The frictional charging process results in a transfer of electrons between the two objects that are rubbed together. Rubbing a balloon on your hair, feet across the carpet Since one object becomes negatively charged and the other positively charged, the two objects will cling together This is why your clothes stick together after being in the dryer!

WAYS TO CHARGE OBJECTS - FRICTION

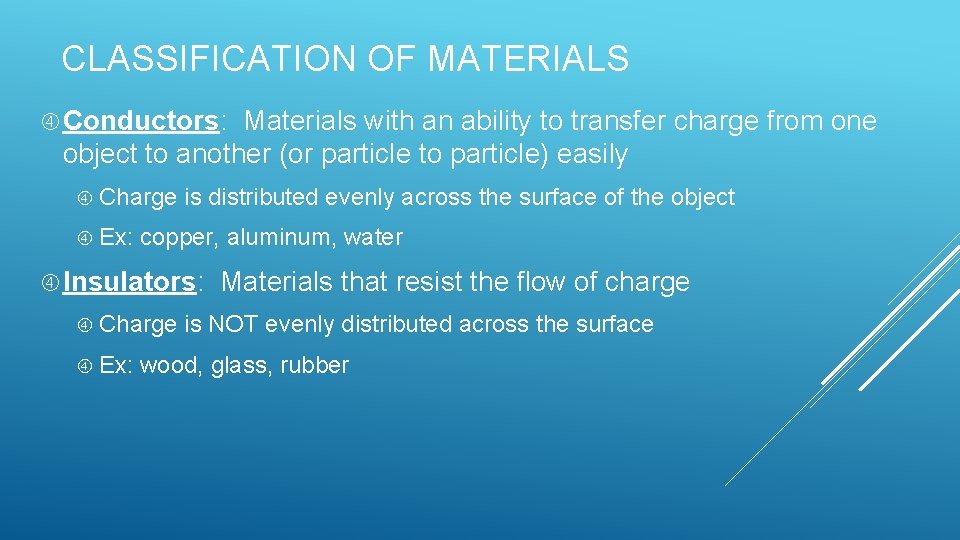
CLASSIFICATION OF MATERIALS Conductors: Materials with an ability to transfer charge from one object to another (or particle to particle) easily Charge is distributed evenly across the surface of the object Ex: copper, aluminum, water Insulators: Materials that resist the flow of charge Charge is NOT evenly distributed across the surface Ex: wood, glass, rubber

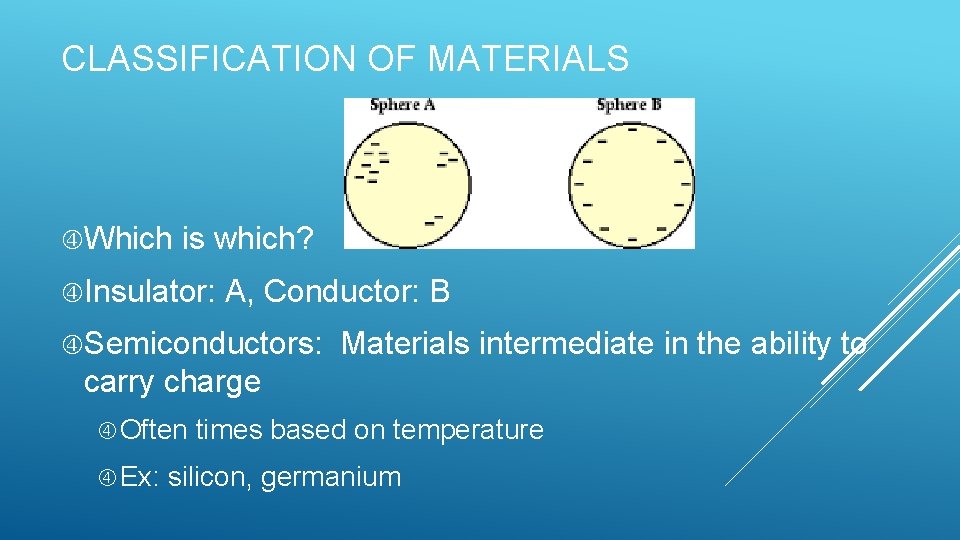
CLASSIFICATION OF MATERIALS Which is which? Insulator: A, Conductor: B Semiconductors: Materials intermediate in the ability to carry charge Often times based on temperature Ex: silicon, germanium

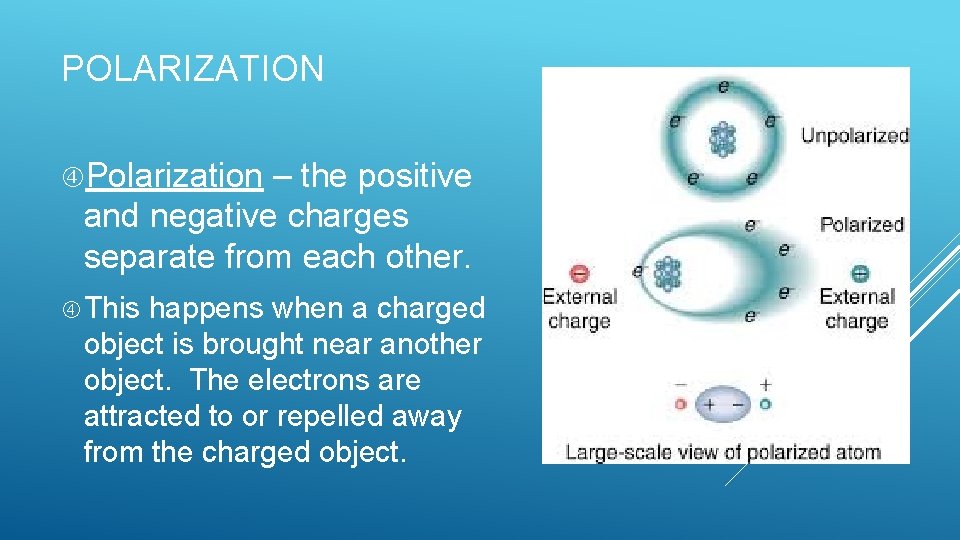
POLARIZATION Polarization – the positive and negative charges separate from each other. This happens when a charged object is brought near another object. The electrons are attracted to or repelled away from the charged object.

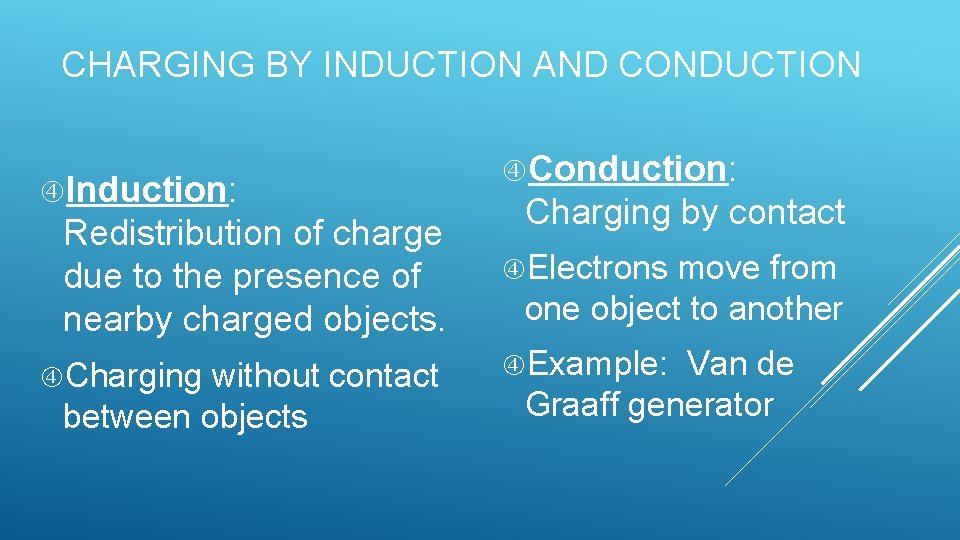
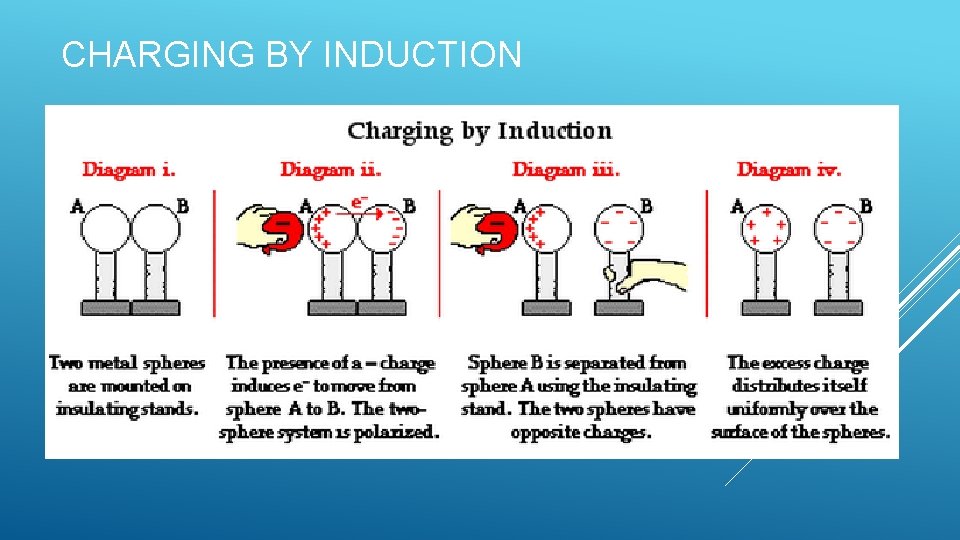
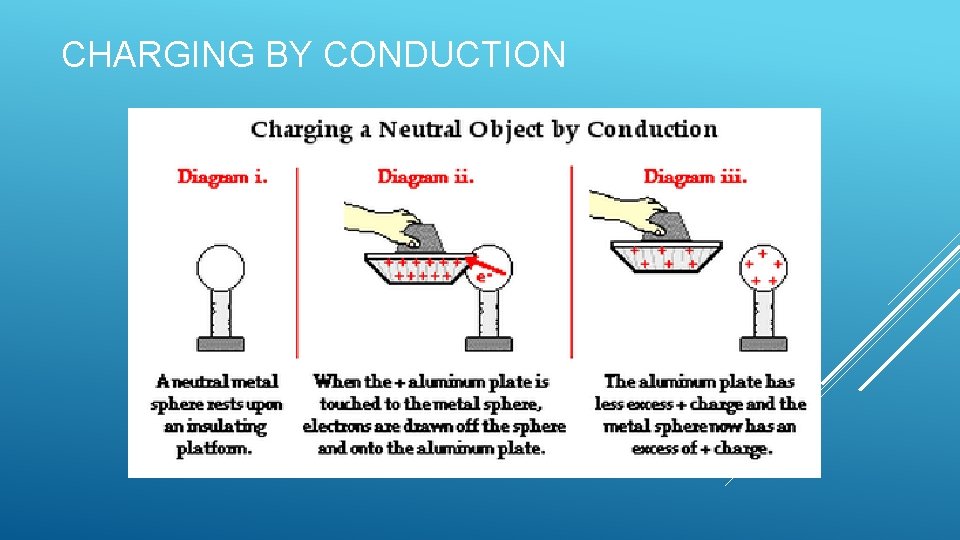
CHARGING BY INDUCTION AND CONDUCTION Induction: Redistribution of charge due to the presence of nearby charged objects. Charging without contact between objects Conduction: Charging by contact Electrons move from one object to another Example: Van de Graaff generator

CHARGING BY INDUCTION

CHARGING BY CONDUCTION

THE LAW OF CONSERVATION OF CHARGE Law of Conservation of Charge – the overall charge in the system is the same before and after the charging process. This is true regardless of the method of charging: friction, induction, or conduction. If 2 electrically neutral objects are rubbed together, and one obtains a charge of +0. 5 C, the other will obtain a charge of -0. 5 C.

ELECTRIC FORCE AND COLOUMB’S LAW The interaction between charged objects is a non- contact force that acts over some distance of separation. Depends on the two charges and the distance between them. Electric force is a vector quantity with both magnitude and direction.

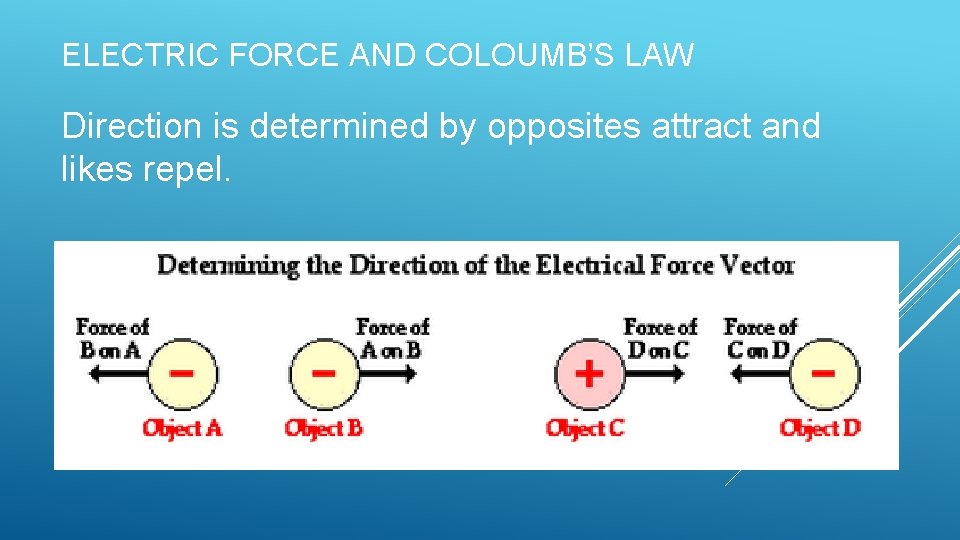
ELECTRIC FORCE AND COLOUMB’S LAW Direction is determined by opposites attract and likes repel.

ELECTRIC FORCE AND COULOMB’S LAW

EXAMPLE PROBLEM #1

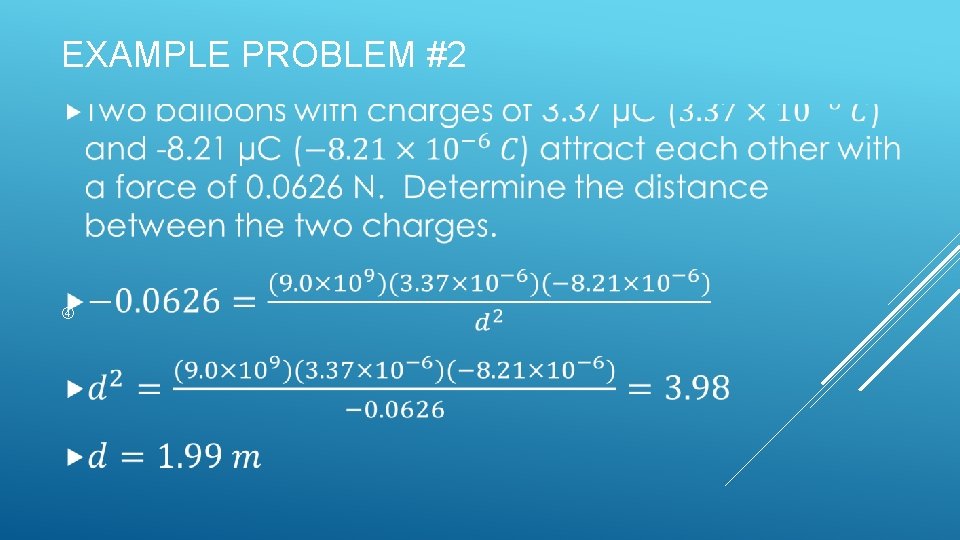
EXAMPLE PROBLEM #2

REVIEW PROBLEM #1

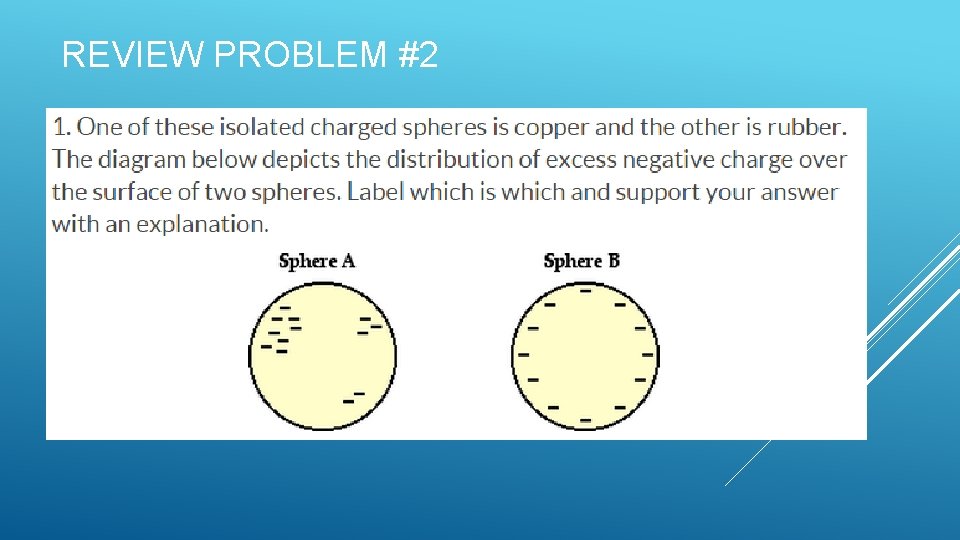
REVIEW PROBLEM #2

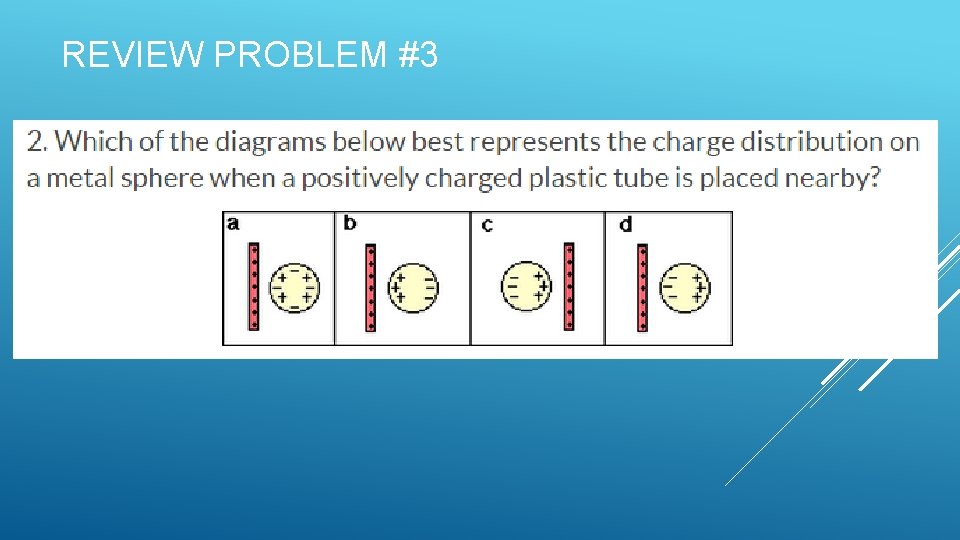
REVIEW PROBLEM #3

REVIEW PROBLEM #4

REVIEW PROBLEM #5

REVIEW PROBLEM #6

REVIEW PROBLEM #7

REVIEW PROBLEM #8 An object that has a charge of 0. 5 C has how many more protons than electrons?

REVIEW PROBLEM #9

ANSWERS

ADDITIONAL REVIEW TOPICS Atomic Structure – Protons, Neutrons, Electrons Electrically neutral vs charged objects Relationship between #excess electrons or protons and charge (be able to calculate charge or from the charge calculate #excess electrons/protons) Charge interactions – attraction/repulsion (what happens between ++, --, +N, -N)

ADDITIONAL REVIEW TOPICS Charging methods: friction, induction, conduction Conductors vs insulators Polarization Conservation of Charge Couloms’ Law