Unit 10 Solutions What is a solution A















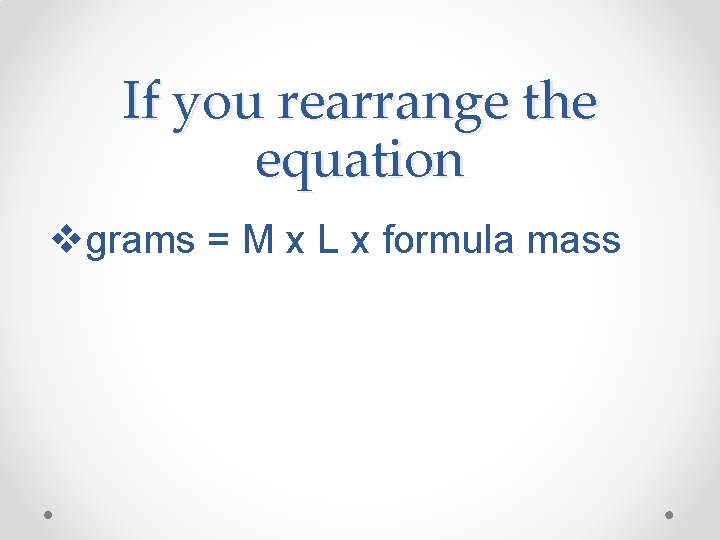




- Slides: 33

Unit 10: Solutions

What is a solution? A homogeneous mixture of a solute dissolved in a solvent. solute solvent

• Solute vs. Solvent https: //phet. colorado. edu/en/simulation/legacy/sugar-and-salt-solutions • Solute: the substance being dissolved (in lesser amount) • Solvent: the medium in which the solute is dissolved (in greater amount) o Water is a very common solvent **A solution refers to both the solute AND solvent • The solute never settles out (it gets evenly mixed)

Solutions can be a combination of different phases Combination Example Solid in a liquid Table salt in water Gas in a liquid Soda Gas in a gas Air Liquid in a gas Humidity Examples: Liquid in a liquid Vinegar

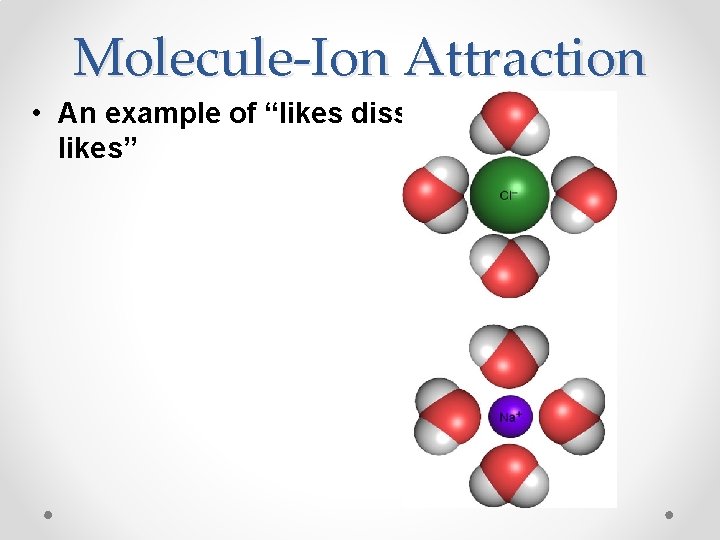
Will all solvents dissolve all solutes? • No- The rule of thumb is “likes dissolve likes” • Polar/ionic solvents dissolve polar/ionic solutes (miscible) • Non-polar solvents dissolve non-polar solutes (miscible) • Polar/non-polar solutes and solvent are immiscible

Molecule-Ion Attraction • An example of “likes dissolve likes”

Solubility • Describes how much of a solute can be dissolved in a given amount of a solvent • Depends on 3 factors: 1) The chemical nature of substances (“likes dissolve likes”) 2) Temperature 3) Pressure 4) Amount of solvent

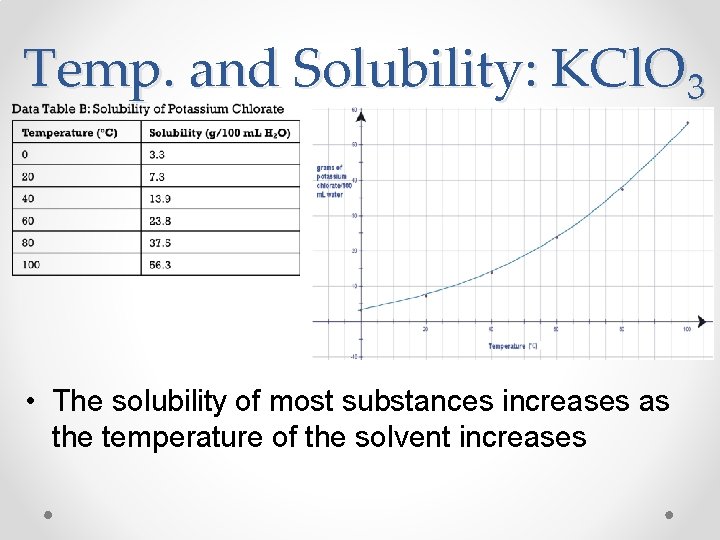
Temp. and Solubility: KCl. O 3 • The solubility of most substances increases as the temperature of the solvent increases

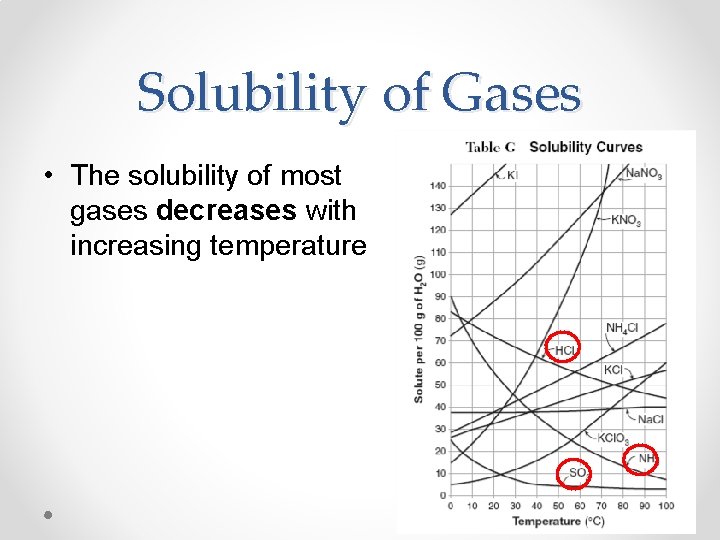
Solubility of Gases • The solubility of most gases decreases with increasing temperature

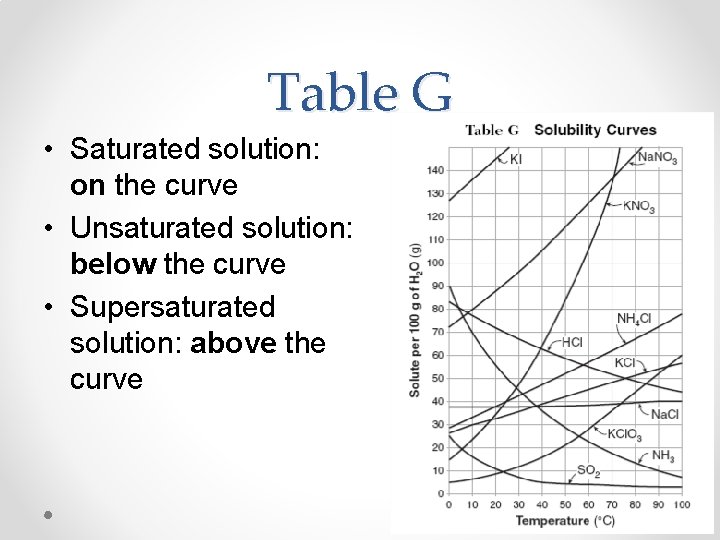
Table G • Saturated solution: on the curve • Unsaturated solution: below the curve • Supersaturated solution: above the curve

Unsaturated Solution • Contains less than the maximum amount of solute allowed under the given conditions • More solute can dissolve

Saturated Solution • Contains the maximum amount of solute dissolved in a solvent • One more crystal will not dissolve (falls to the bottom)

Supersaturated Solution • https: //www. youtube. com/watch? v=BLq 5 Nib w. V 5 g • Contains more than the maximum amount of solute allowed under the given conditions • One more crystal will cause other solute particles to come out of solution rapidly • Ex) rock candy

Notes Quiz! 1) Define saturated solution 2) What are the 4 factors that affect solubility? 3) What happens to the solubility of a gas with increasing temperature? 4) Define supersaturated solution

Solubility and Pressure • Changes in pressure only influence the solubility of gases • Gas solubility increases as the pressure of the gas above the solution increases

When are gases most soluble? • Gases are most soluble under conditions of LOW temperature and HIGH pressure

Complete/Net Ionic Equations Spectator ion: ion that appears on both sides of a complete ionic equation **Is not directly involved in the reaction Net ionic equation: shows ONLY those particles that are directly involved in a chemical change

Complete/Net Ionic Equations Spectator ion: ion that appears on both sides of a complete ionic equation **Is not directly involved in the reaction Net ionic equation: shows ONLY those particles that are directly involved in a chemical change

Concentration • Concentration: measures the amount of solute dissolved in a given quantity of solvent o Dilute solution: contains a small amount of solute o Concentrated solution: contains a large amount of solute https: //phet. colorado. edu/sims/html/concentration/la test/concentration_en. html

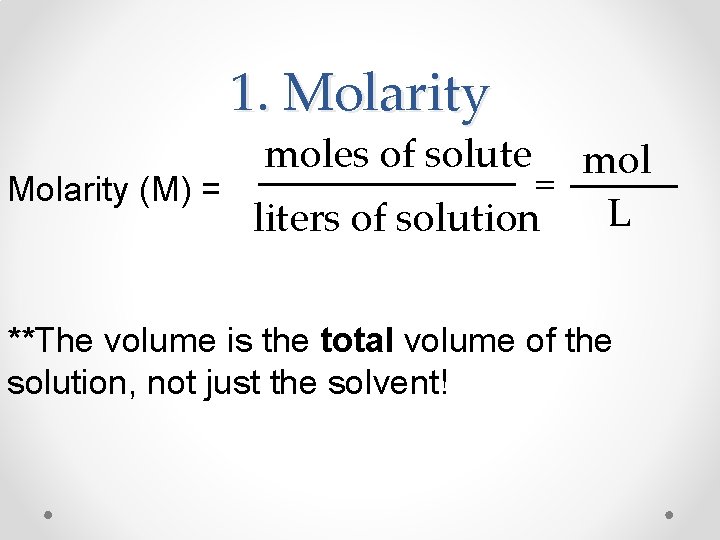
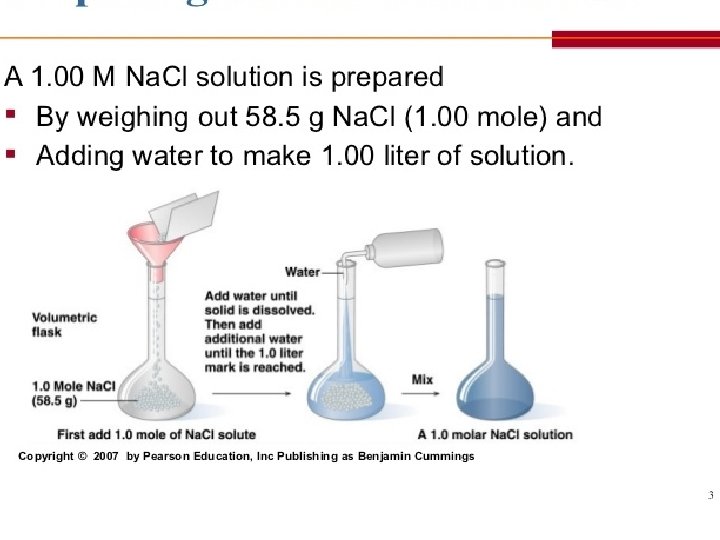
1. Molarity moles of solute mol = Molarity (M) = L liters of solution **The volume is the total volume of the solution, not just the solvent!
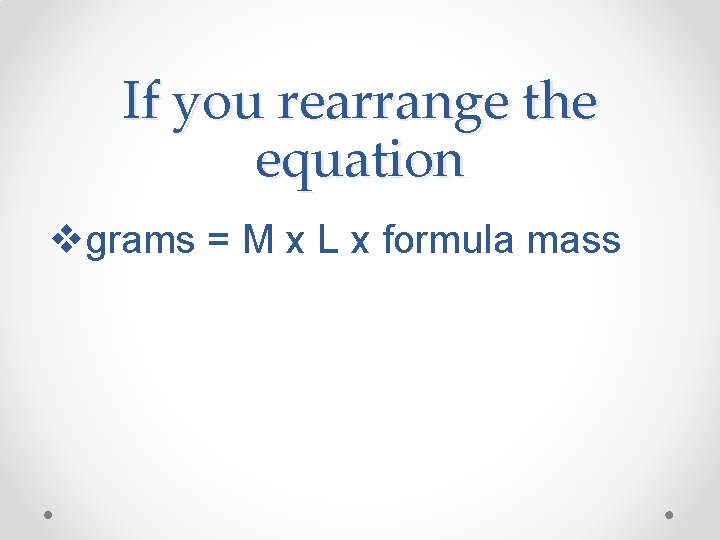
If you rearrange the equation vgrams = M x L x formula mass


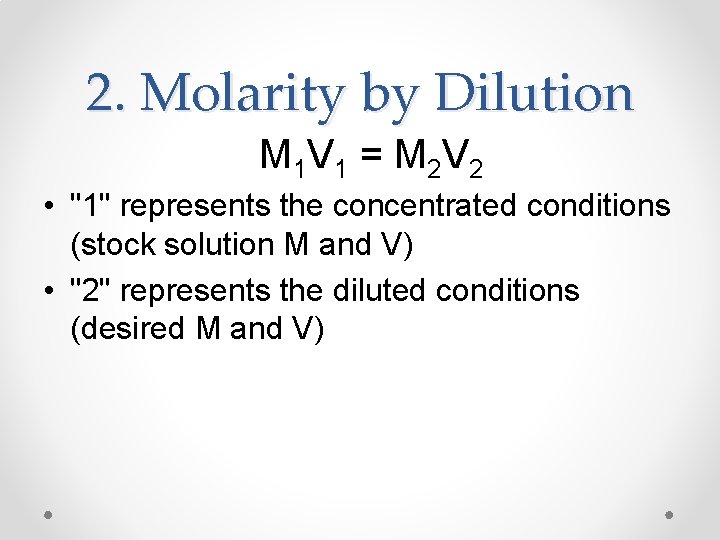
2. Molarity by Dilution M 1 V 1 = M 2 V 2 • "1" represents the concentrated conditions (stock solution M and V) • "2" represents the diluted conditions (desired M and V)

3. % by mass and % by volume mass of solute % by mass = x 100 mass of solution volume of solute % by volume = x 100 volume of solution

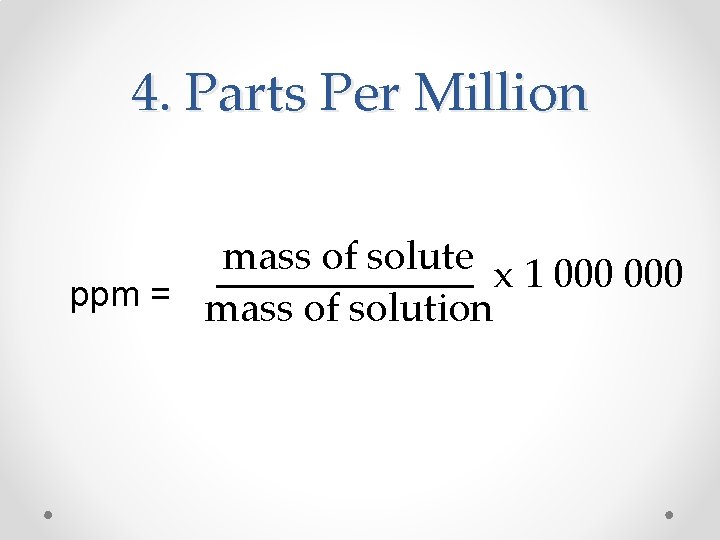
4. Parts Per Million mass of solute x 1 000 ppm = mass of solution

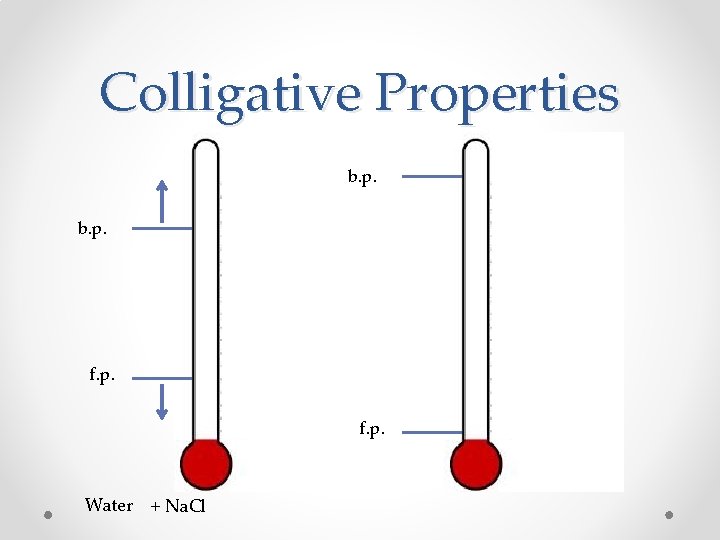
Colligative Properties • A property that depends ONLY on the number of solute particles, and not on their identity • 2 important colligative properties: o Freezing-point depression o Boiling-point elevation

Colligative Properties b. p. f. p. Water + Na. Cl

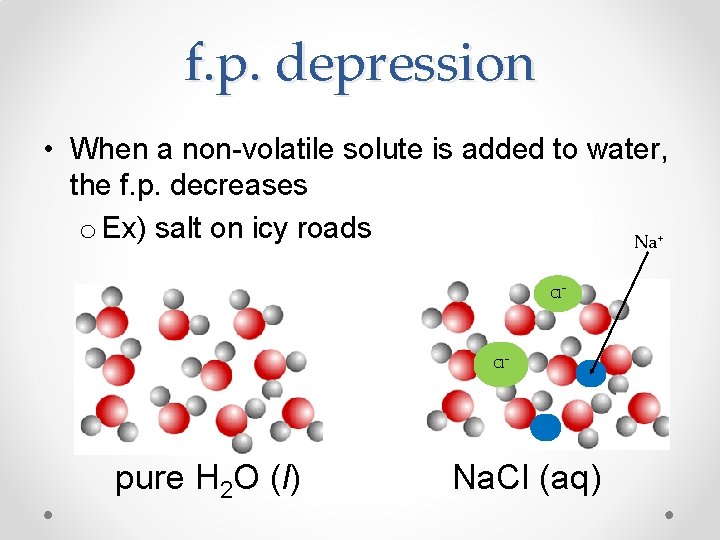
f. p. depression • When a non-volatile solute is added to water, the f. p. decreases o Ex) salt on icy roads Na + Cl- pure H 2 O (l) Na. Cl (aq)

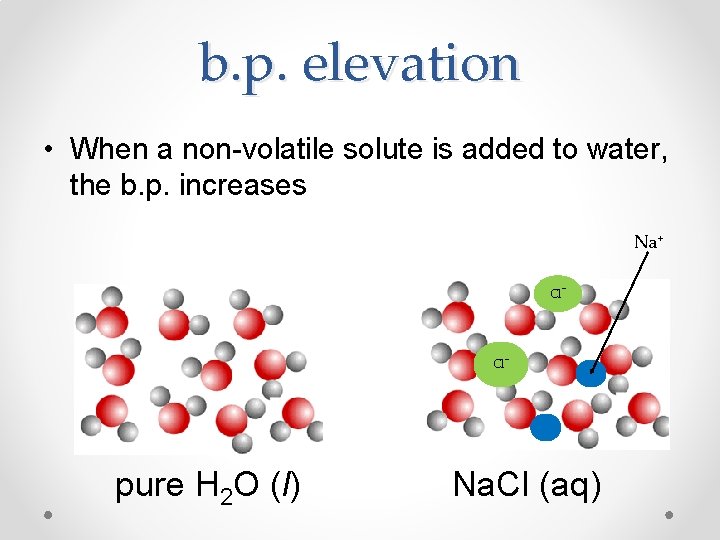
b. p. elevation • When a non-volatile solute is added to water, the b. p. increases Na+ Cl- pure H 2 O (l) Na. Cl (aq)

Amount of temp. change depends on concentration • The more concentrated the solution, the higher the b. p. and the lower the f. p. Which solution would have the higher boiling point? A: 2 M Na. Cl (aq) B: 3 M Na. Cl (aq)

Challenge Question Which solution has the lower freezing point: 1. 0 M Na. Cl (aq) or 1. 0 M C 12 H 22 O 11(aq)?

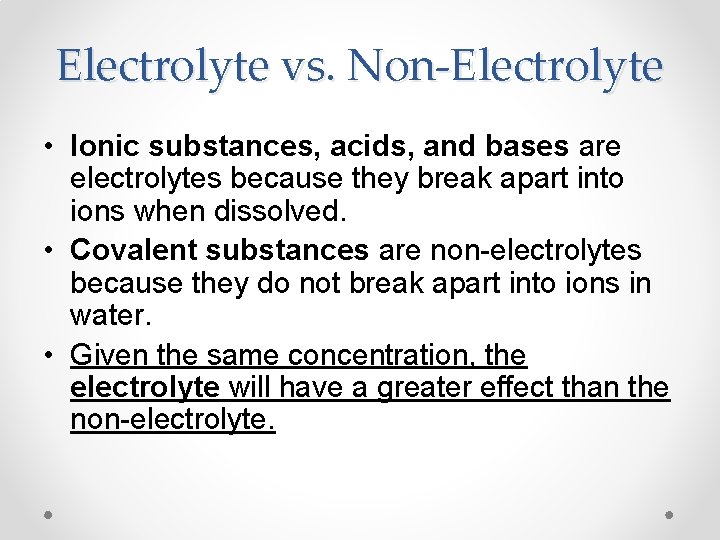
Electrolyte vs. Non-Electrolyte • Ionic substances, acids, and bases are electrolytes because they break apart into ions when dissolved. • Covalent substances are non-electrolytes because they do not break apart into ions in water. • Given the same concentration, the electrolyte will have a greater effect than the non-electrolyte.

More on Electrolytes. . . Which solution has a higher boiling point? A: 1. 5 M Na. Cl (aq) B: 1. 5 M Ca. Cl 2 (aq) • The greater the number of dissociated ions, the higher the b. p. and the lower the f. p.