Unit 10 Solutions 10 1 Mixtures and Solubility

Unit 10: Solutions • • • 10. 1 Mixtures and Solubility 10. 2 Concentration 10. 3 Colligative Properties

10. 1 Mixtures and Solubility • Learning Targets • Compare and contrast suspensions, colloids, and solutions. • Determine the solubility of a given substance. • Describe factors that affect solubility.

10. 1 Mixtures and Solubility • • Review: So far, we have studied mostly pure substances…

10. 1 Mixtures and Solubility • Substance • Pure material, one chemical formula • Can be elements or compounds

10. 1 Mixtures and Solubility • Mixture: • • Combination or blend of 2 or more substances Can be physically separated

10. 1 Mixtures and Solubility • 2 types of mixtures: • Heterogeneous mixture: • Not evenly mixed, visible differences

10. 1 Mixtures and Solubility • Homogeneous mixture: • Evenly mixed, no visible differences

10. 1 Mixtures and Solubility • Suspension • • • A mixture that is heterogeneous. Particles size is large. Will separate (settle out) without stirring. • Examples: • • • Mud in water. Ash in air Salad dressing

10. 1 Mixtures and Solubility • Colloid • • A stable homogenous mixture that is cloudy Medium particle size. Particles do not settle out. Tyndall effect • Light scatters when particles are hit • Examples: • • • Milk Fog Whipped cream Blood Glass

10. 1 Mixtures and Solubility • Solution • A stable homogenous mixture that is clear • Examples: • • Particles are small. • • • No Tyndall effect Vinegar Salt Water Soda Air Metal Alloys

10. 1 Mixtures and Solubility • Dissolve • • Solvent • • Substance mixes uniformly to form a solution Larger amount that does dissolving. Solute • Dissolves in the solvent

10. 1 Mixtures and Solubility • Solubility: • • Ability of a solute to dissolve (mix) Soluble: • Solute will dissolve in a solvent • • Na. Cl in water Insoluble: • Solute will not dissolve in a solvent • Oil in water
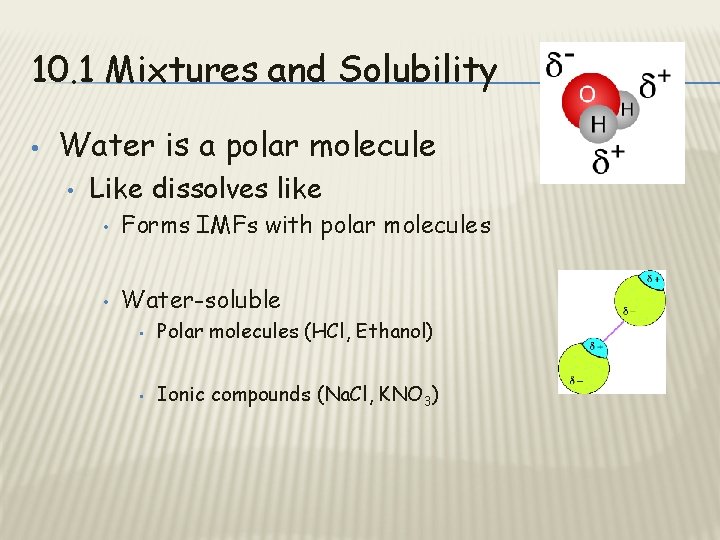
10. 1 Mixtures and Solubility • Water is a polar molecule • Like dissolves like • Forms IMFs with polar molecules • Water-soluble • Polar molecules (HCl, Ethanol) • Ionic compounds (Na. Cl, KNO 3)

10. 1 Mixtures and Solubility • Water-insoluble • • Nonpolar molecules (CH 4, I 2) Only nonpolar solvents (paint thinner) dissolve nonpolar solutes (paint)
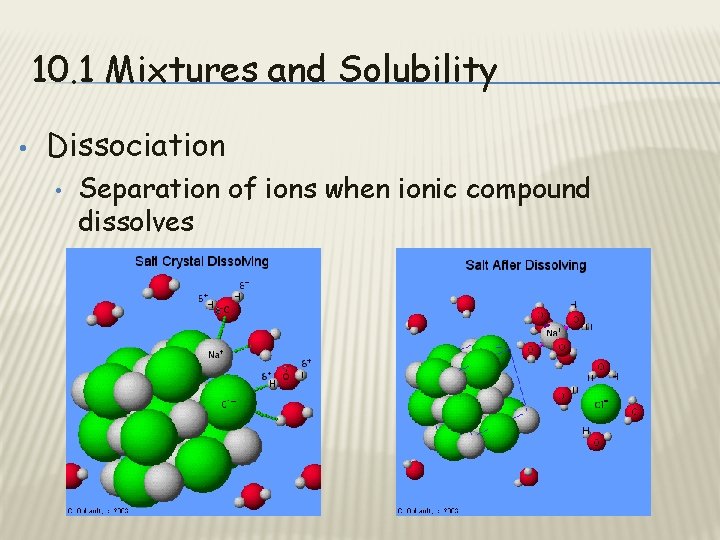
10. 1 Mixtures and Solubility • Dissociation • Separation of ions when ionic compound dissolves

10. 1 Mixtures and Solubility • • • Amount of solute that will dissolve under certain conditions Factors Affecting Solubility 1. Substance Type • Nonpolar substances dissolve in nonpolar solvents Polar/ionic substances dissolve in polar solvents • “Like dissolves like” •
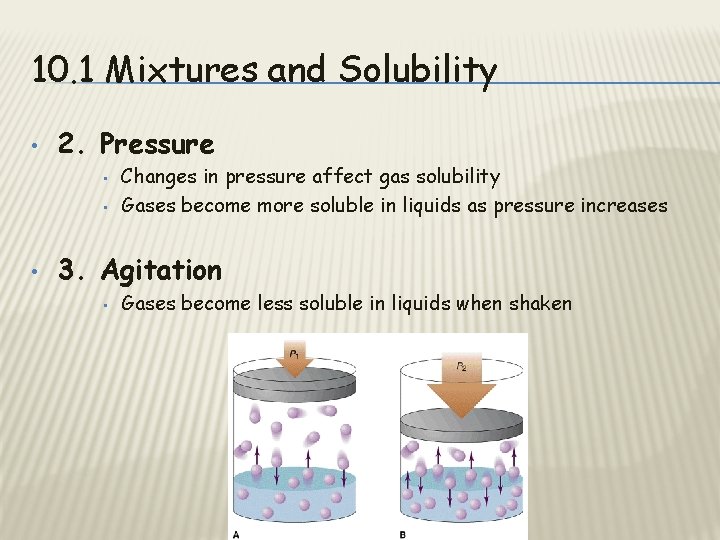
10. 1 Mixtures and Solubility • 2. Pressure • • • Changes in pressure affect gas solubility Gases become more soluble in liquids as pressure increases 3. Agitation • Gases become less soluble in liquids when shaken
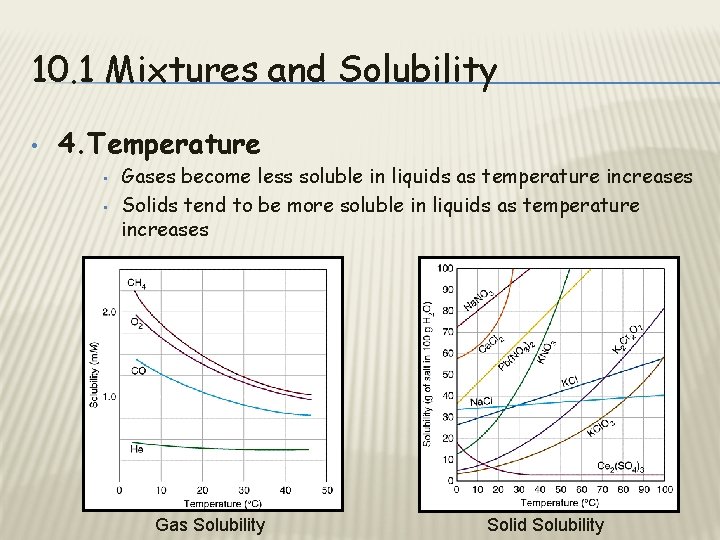
10. 1 Mixtures and Solubility • 4. Temperature • • Gases become less soluble in liquids as temperature increases Solids tend to be more soluble in liquids as temperature increases Gas Solubility Solid Solubility

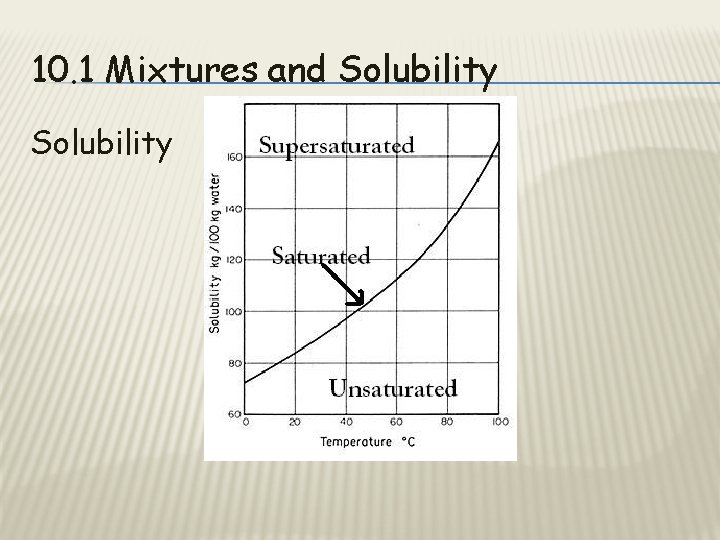
10. 1 Mixtures and Solubility • Unsaturated: • • Solution can dissolve more solute. Saturated: • Maximum amount of solute a solution can dissolve

10. 1 Mixtures and Solubility • Supersaturated: • • Contains more dissolved solute than a saturated solution. https: //www. youtube. com/watch? v=j. MBIS 2 e Mmm 4
10. 1 Mixtures and Solubility
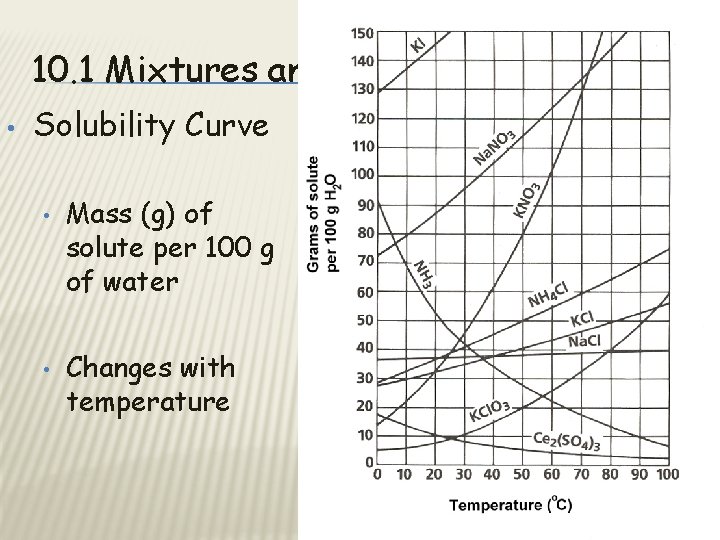
10. 1 Mixtures and Solubility • Solubility Curve • • Mass (g) of solute per 100 g of water Changes with temperature
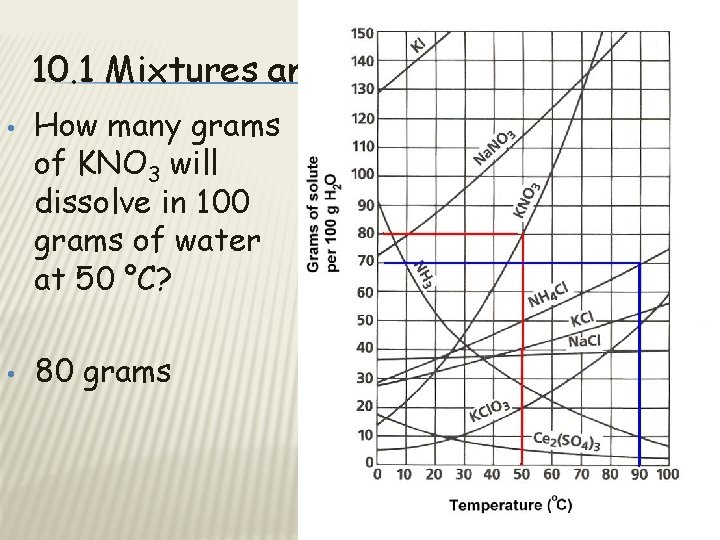
10. 1 Mixtures and Solubility • • How many grams of KNO 3 will dissolve in 100 grams of water at 50 °C? 80 grams
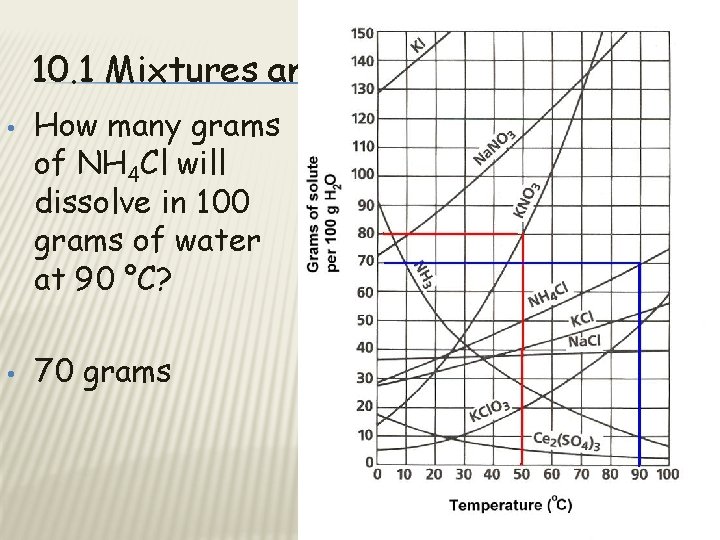
10. 1 Mixtures and Solubility • • How many grams of NH 4 Cl will dissolve in 100 grams of water at 90 °C? 70 grams

10. 1 Mixtures and Solubility • How fast will a solute dissolve? • Surface area • • Increase surface area (grind up), dissolves faster Temperature • Increase temperature, dissolve faster

10. 1 Mixtures and Solubility • Stirring • • Stir/agitate, dissolve faster Substance • Different substances have different strengths of IMFs • • Stronger IMFs = dissolve slower Weaker IMFs = dissolve faster


10. 2 Concentration • Learning Targets • Calculate concentrations of solutions using molarity.

10. 2 Concentration • Concentration: • • Concentrated: • • Amount of solute that is dissolved in a solution A lot of solute dissolved (high concentration) Dilute • Not a lot of solute dissolved (low concentration)
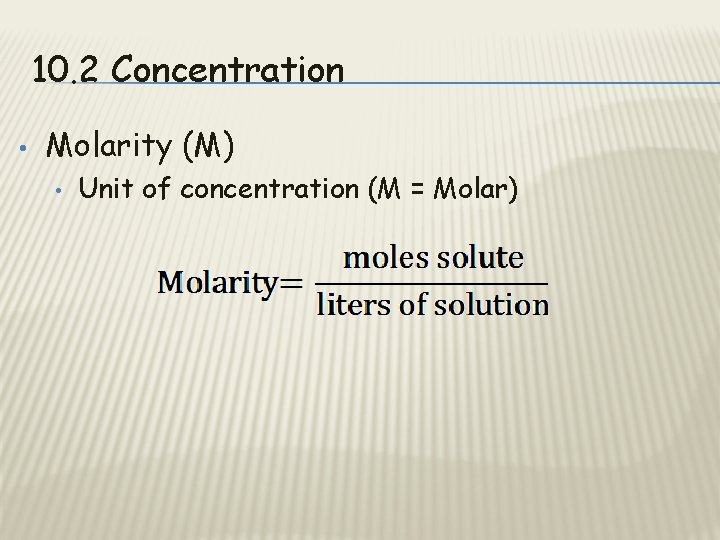
10. 2 Concentration • Molarity (M) • Unit of concentration (M = Molar)

10. 2 Concentration • #1. What is the molarity of a solution that contains 20 grams of sodium hydroxide in 125 m. L of solution?

10. 2 Concentration • #2. How many moles of HCl are dissolved in 722 m. L of a 0. 30 M solution?

10. 2 Concentration • #3. How many grams of Na. Cl are needed to produce 0. 60 L of a 0. 22 M solution?

10. 2 Concentration • #4. What volume of a 0. 25 M solution will contain 0. 178 moles of KI?


10. 4 Colligative Properties • Learning Targets • Calculate concentrations of solutions using molarity. • Use concentration to predict the colligative properties of a solution.

10. 4 Colligative Properties • Colligative Property • • Property of solution that depends only on the amount of solute A mixture will have different characteristics than the pure solvent

10. 4 Colligative Properties • Boiling Point Elevation • • • Adding a solute increases the boiling point (temperature) Salt in water Antifreeze in engine

10. 4 Colligative Properties • Boiling Point Elevation • • Temperature needed to boil increases ΔTB : change in boiling temperature KB : constant for a solvent (reference sheet) i : strength (given in problem)

10. 4 Colligative Properties • • #1. What is the boiling point elevation of water in a solution that contains 20 g Na. OH (i=2) dissolved in 200 g of water? #2. What is the boiling point of the solution?

10. 4 Colligative Properties • • #3. What is the boiling point elevation of an ether solution that contains 10 g I 2 (i=1) dissolved in 100 g of ether? #4. What is the boiling point of the solution?

10. 4 Colligative Properties • Freezing Point Depression • • Adding a solute lowers the freezing point (temperature) Salt on icy roads Ice cream Antifreeze in engine
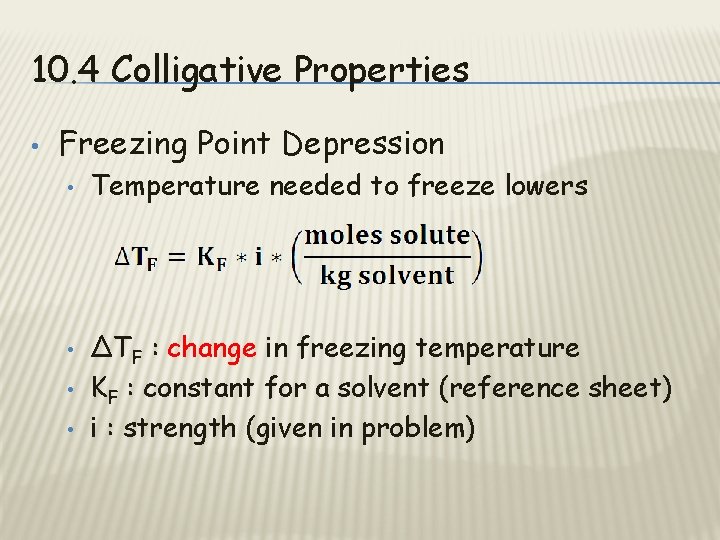
10. 4 Colligative Properties • Freezing Point Depression • • Temperature needed to freeze lowers ΔTF : change in freezing temperature KF : constant for a solvent (reference sheet) i : strength (given in problem)

10. 4 Colligative Properties • • #5. What is the freezing point depression of water in a solution that contains 50 g Al. Cl 3 (i=4) dissolved in 1. 2 kg of water? #6. What is the freezing point of the solution?

10. 4 Colligative Properties • #7. What is the freezing point of a solution that contains 50 g of CO 2 (i = 1) dissolved in 1. 4 kg of phenol?
- Slides: 45