Unit 10 Oxidationreduction Redox Chapter 19 Oxidation charges










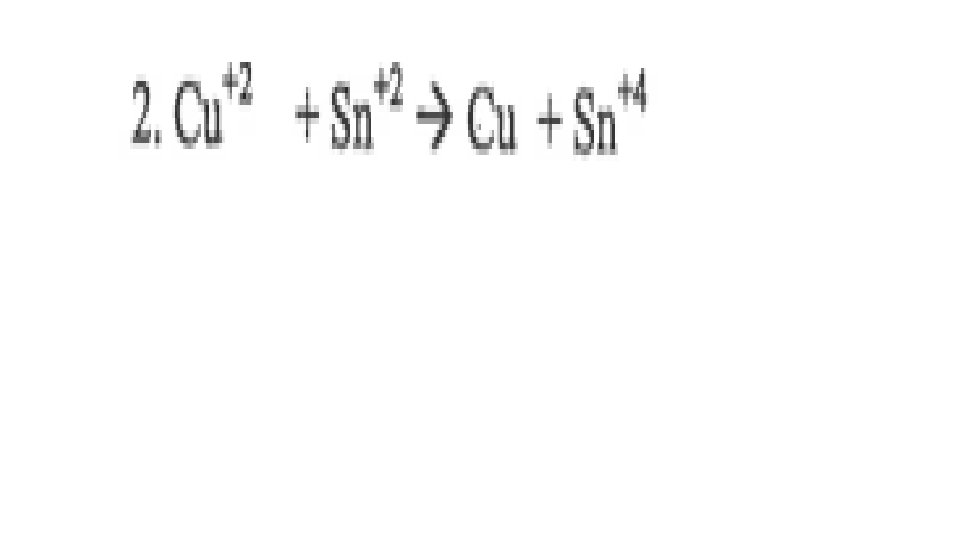
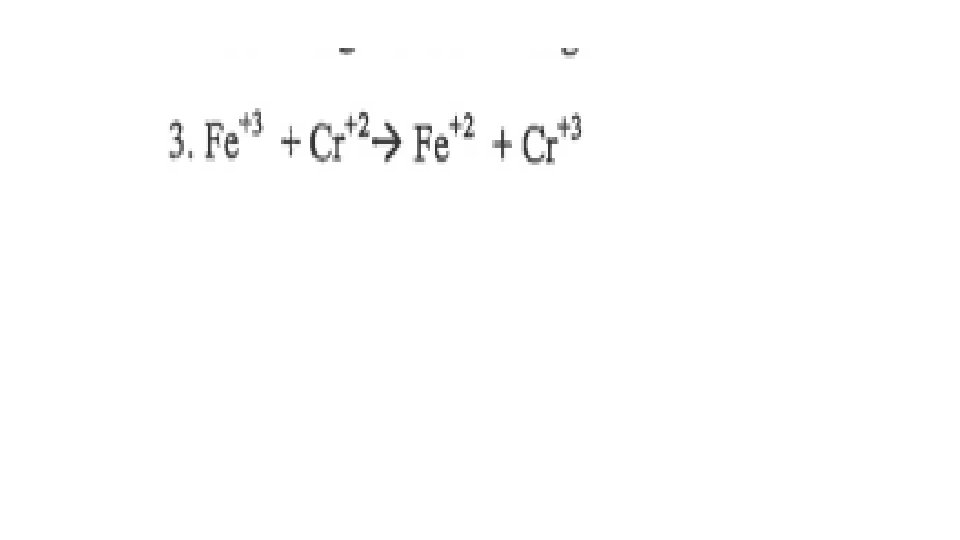





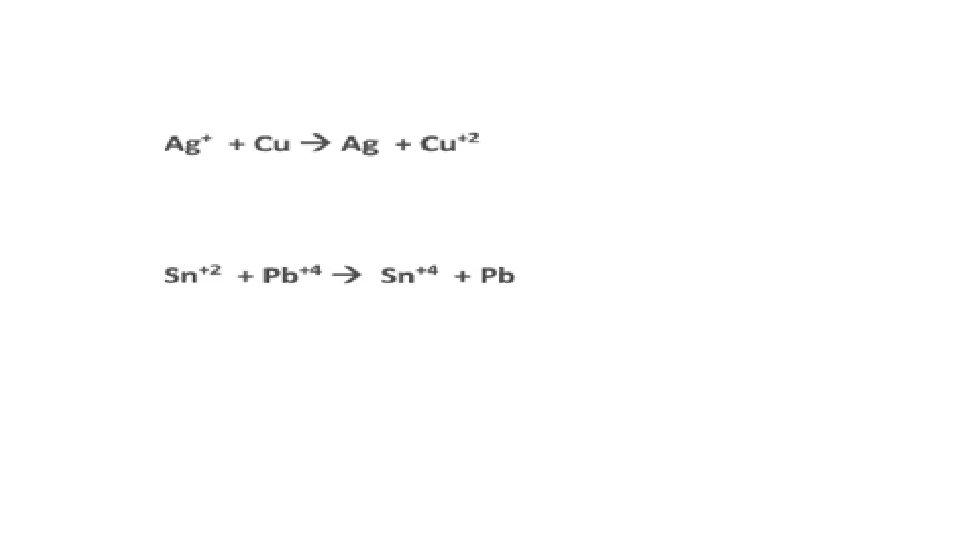





- Slides: 52

Unit 10 Oxidation-reduction (Redox) Chapter 19 Oxidation #: charges given to an atom of an element Where can we find the oxidation numbers for the elements? The periodic table 1) Uncombined elements(elements not bonded to another element) have an oxidation number of zero. Examples: Al 0(s) , Cl 02(g) , Na 0(s) , H 02(g)

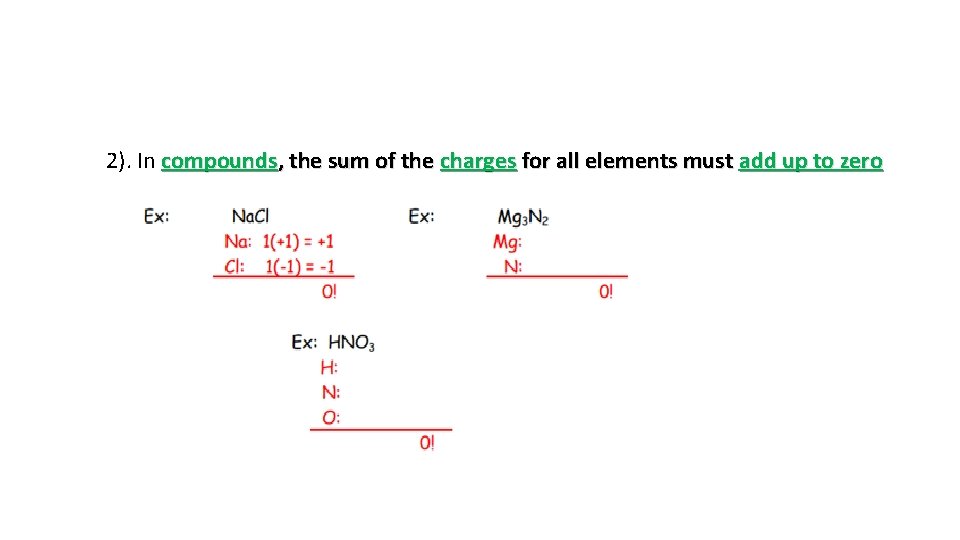
2). In compounds, the sum of the charges for all elements must add up to zero

• The oxidation # is the number inside the parenthesis. It is the charge on one atom of that element! **Tric. K: You keep the polyatomics ions together and use the charge from Table E to determine the oxidation numbers for those elements. ***Remember that we almost always write (+) element first and the ( -) element last. EXAMPLE: HCl EXCEPTION to this rule: NH 3

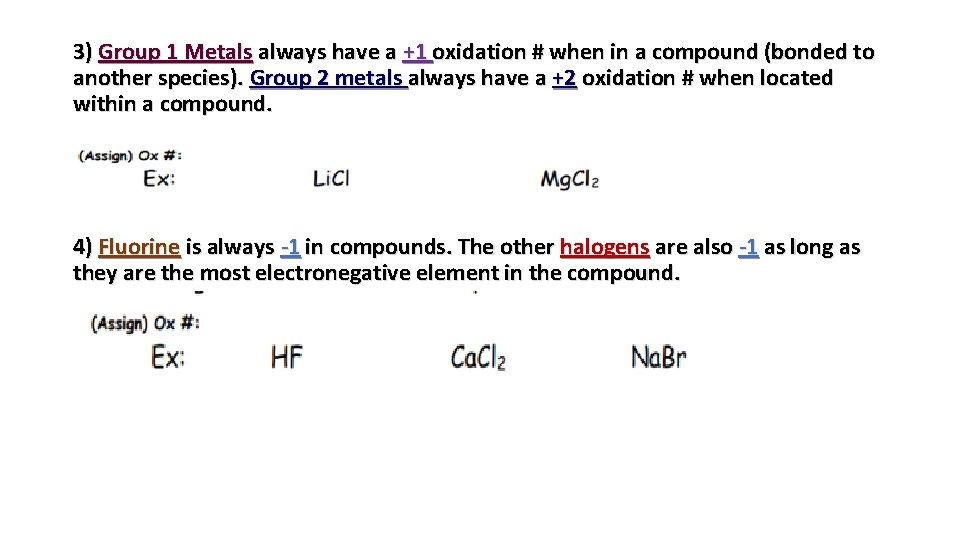
3) Group 1 Metals always have a +1 oxidation # when in a compound (bonded to another species). Group 2 metals always have a +2 oxidation # when located within a compound. 4) Fluorine is always -1 in compounds. The other halogens are also -1 as long as they are the most electronegative element in the compound.

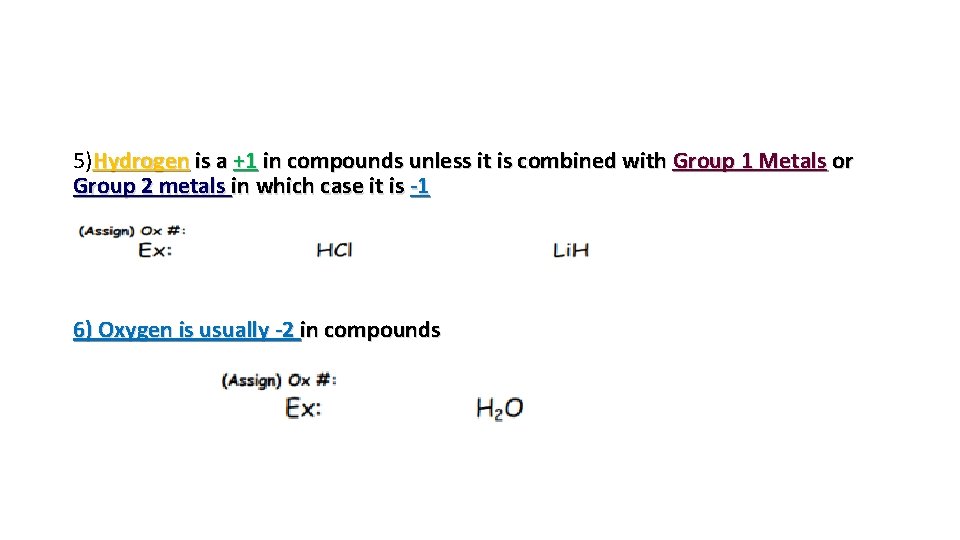
5)Hydrogen is a +1 in compounds unless it is combined with Group 1 Metals or Group 2 metals in which case it is -1 6) Oxygen is usually -2 in compounds

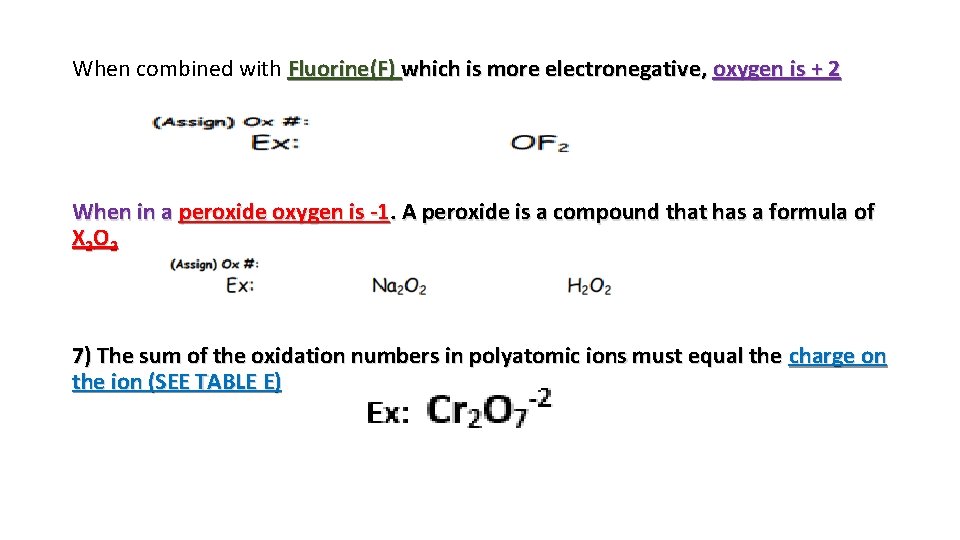
When combined with Fluorine(F) which is more electronegative, oxygen is + 2 When in a peroxide oxygen is -1. A peroxide is a compound that has a formula of X 2 O 2 7) The sum of the oxidation numbers in polyatomic ions must equal the charge on the ion (SEE TABLE E)


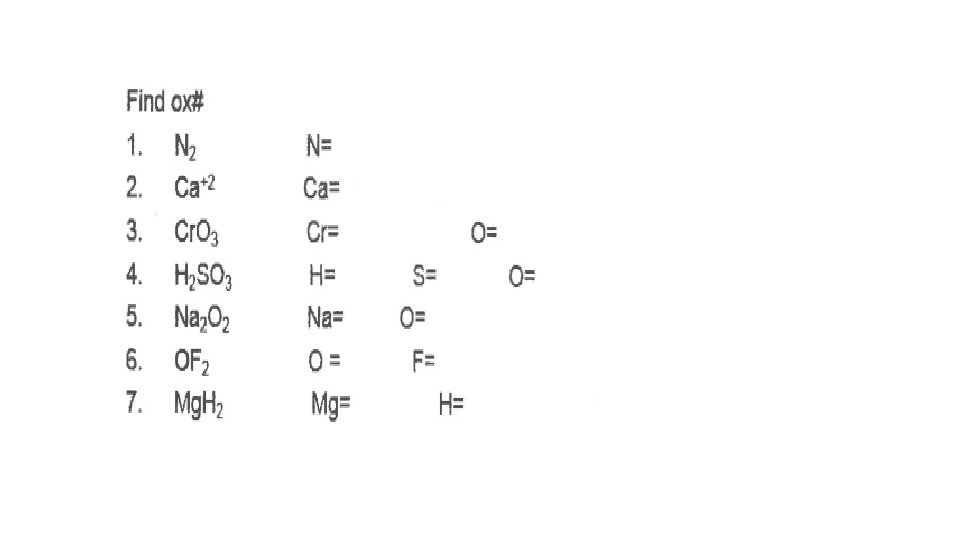
1. HCl H= +1 Cl=-1 2. KNO 3 K=+1 N=+5 O=-2 3. OHO=-2 H=+1 4. Mg 3 N 2 Mg=+2 N=-3 5. KCl. O 3 K=+1 Cl=+5 O=-2 6. Al(NO 3)3 Al=+3 N=+5 O=-2 7. S 8 S=0 8. H 2 O 2 H=+1 O=-1 9. Pb. O 2 Pb=+4 O=-2 10. Na. HSO 4 Na=+1 H=+1 S=+6 O=-2

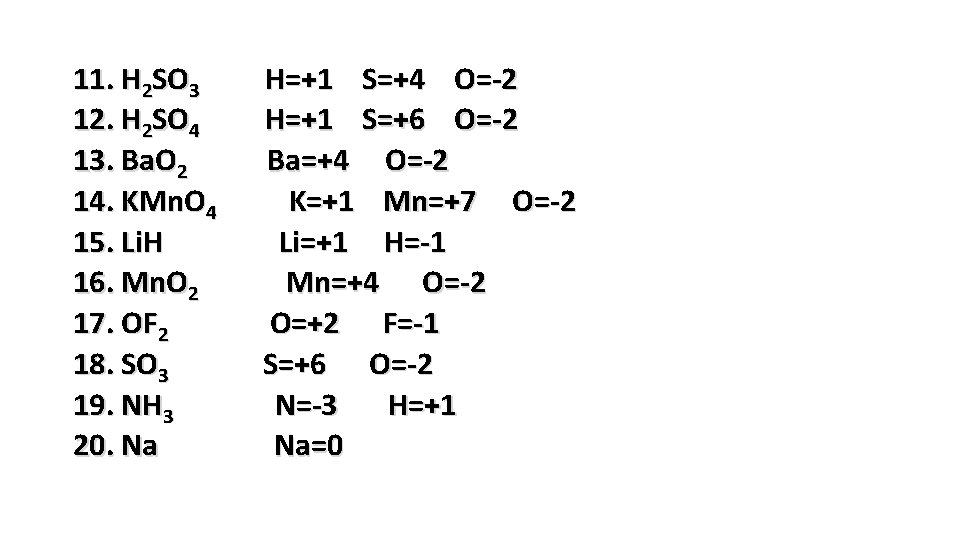
11. H 2 SO 3 12. H 2 SO 4 13. Ba. O 2 14. KMn. O 4 15. Li. H 16. Mn. O 2 17. OF 2 18. SO 3 19. NH 3 20. Na H=+1 S=+4 O=-2 H=+1 S=+6 O=-2 Ba=+4 O=-2 K=+1 Mn=+7 O=-2 Li=+1 H=-1 Mn=+4 O=-2 O=+2 F=-1 S=+6 O=-2 N=-3 H=+1 Na=0

Find the oxidation numbers for: Li. Na. KH 2 SO 3 Page 19 1. A 2. D 3. B 4. B 5. A 6. B 7. A 8. B

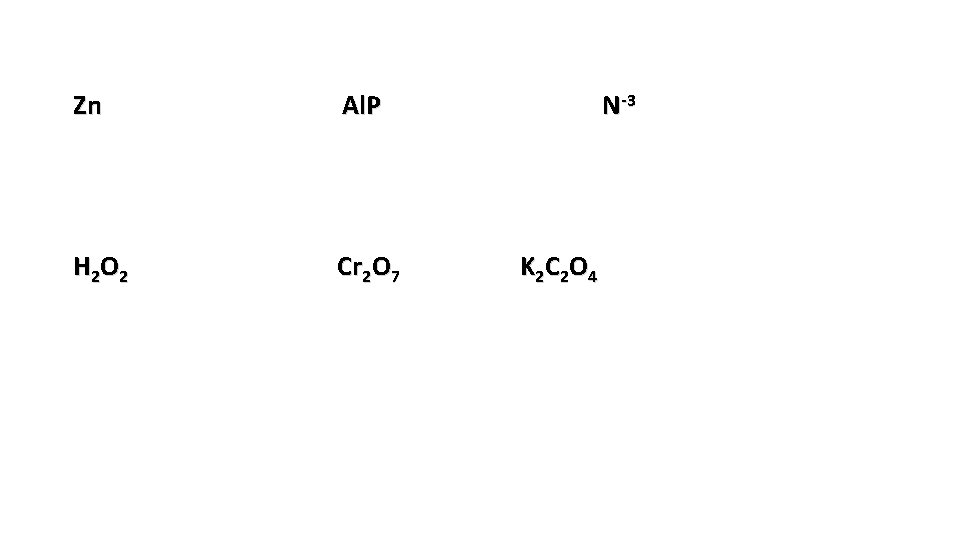
Zn Al. P H 2 O 2 Cr 2 O 7 N-3 K 2 C 2 O 4

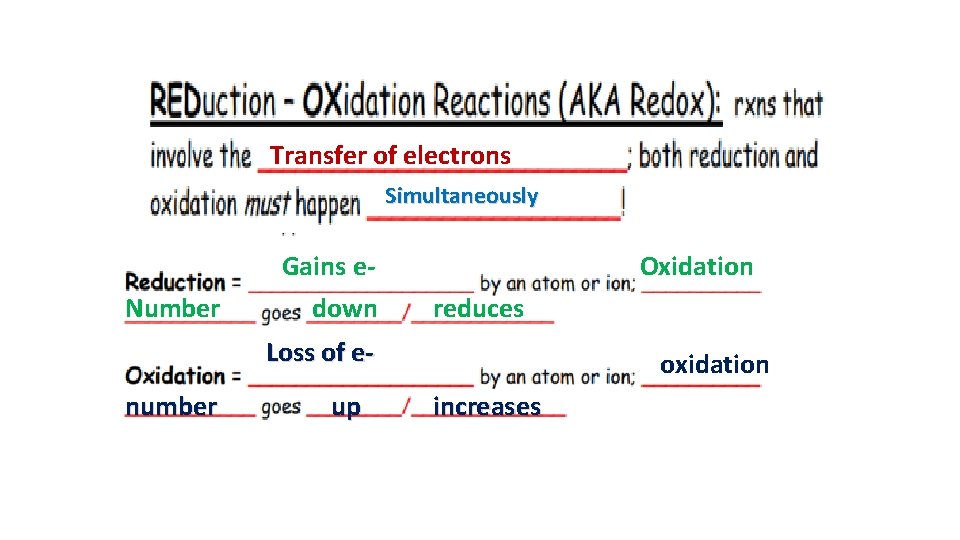
Transfer of electrons Simultaneously Number Gains edown Oxidation reduces Loss of enumber up oxidation increases

Lose e- oxidation Gain e- reduction

desire lose gain mutal simultaneous gains loses

LAW OF CONSERVATION OF CHARGE

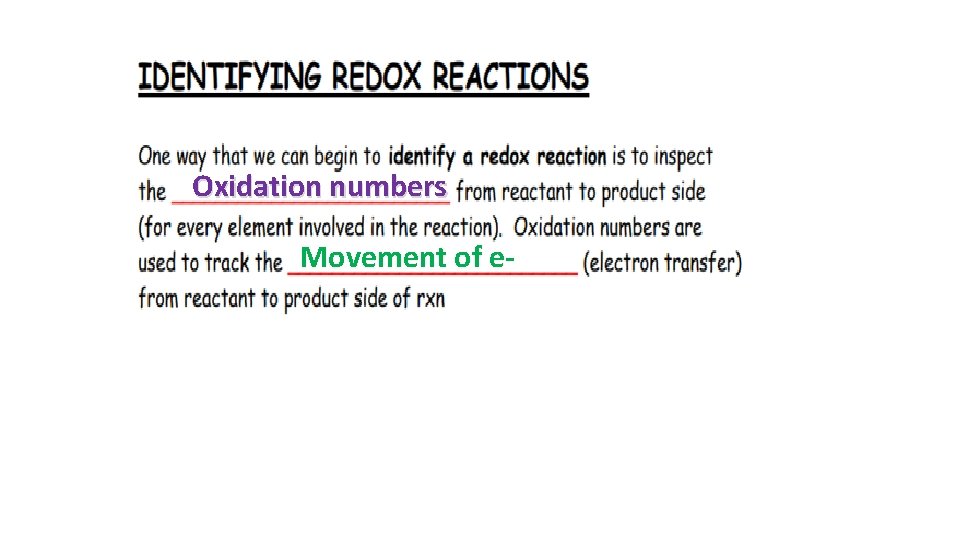
Oxidation numbers Movement of e-

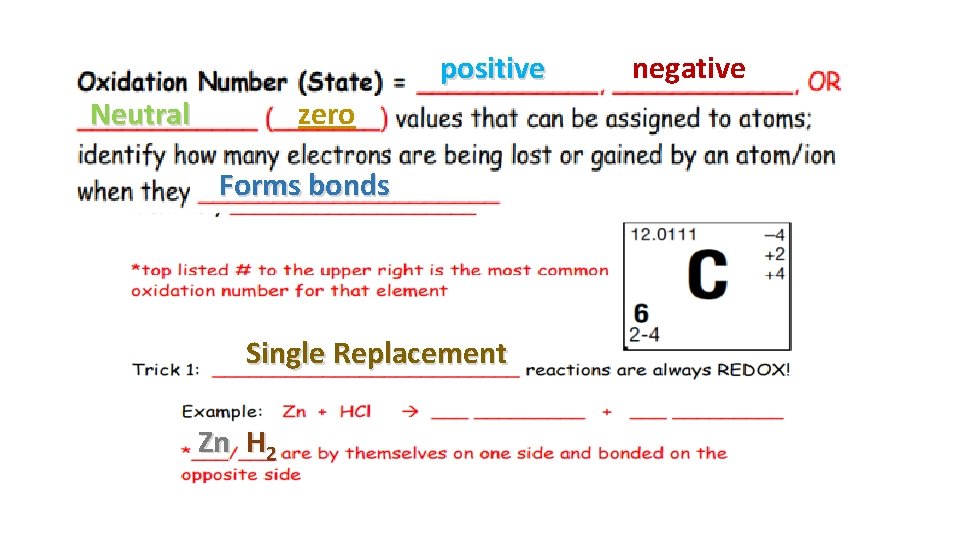
positive Neutral zero Forms bonds Single Replacement Zn H 2 negative


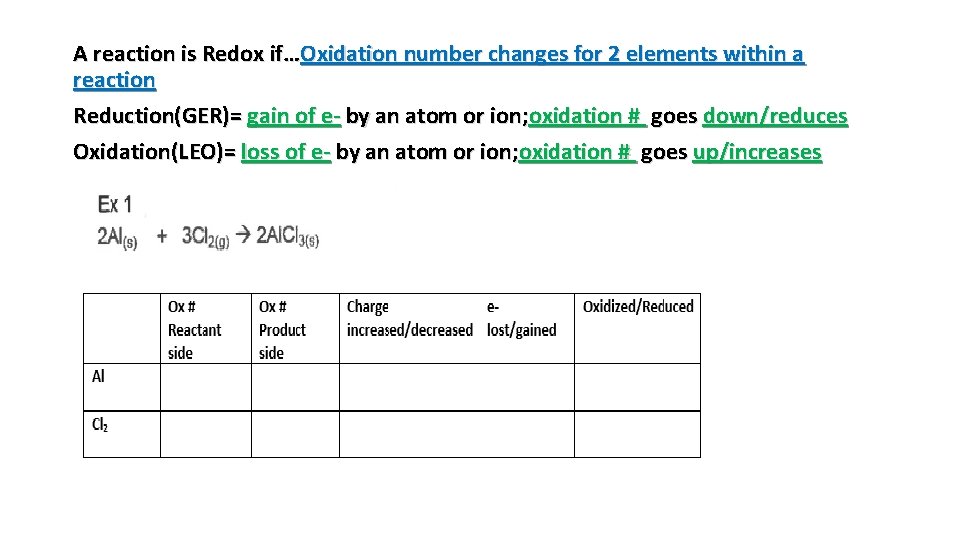
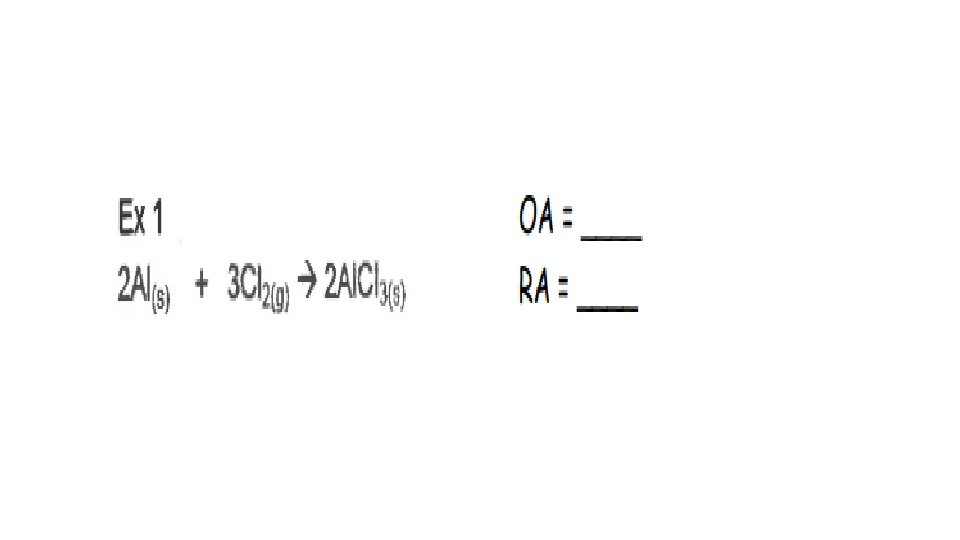
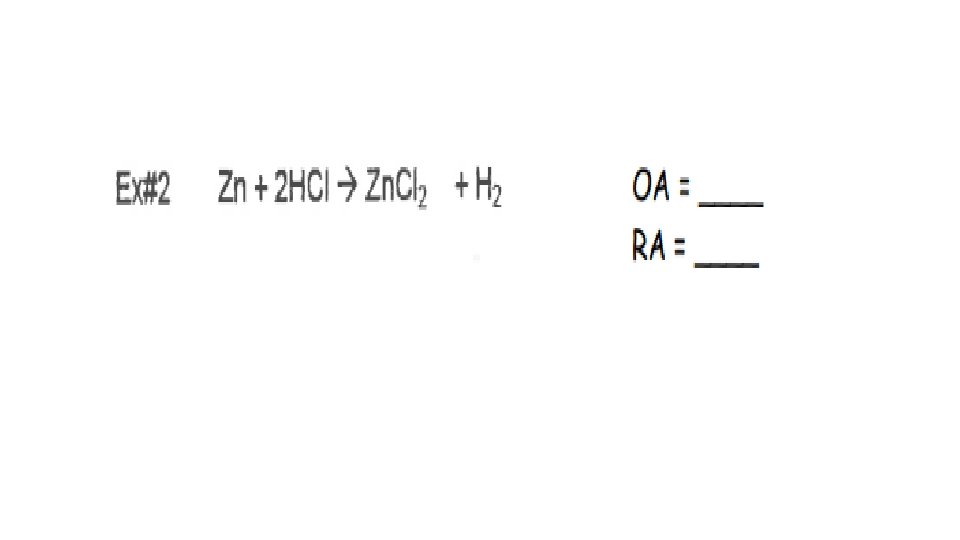
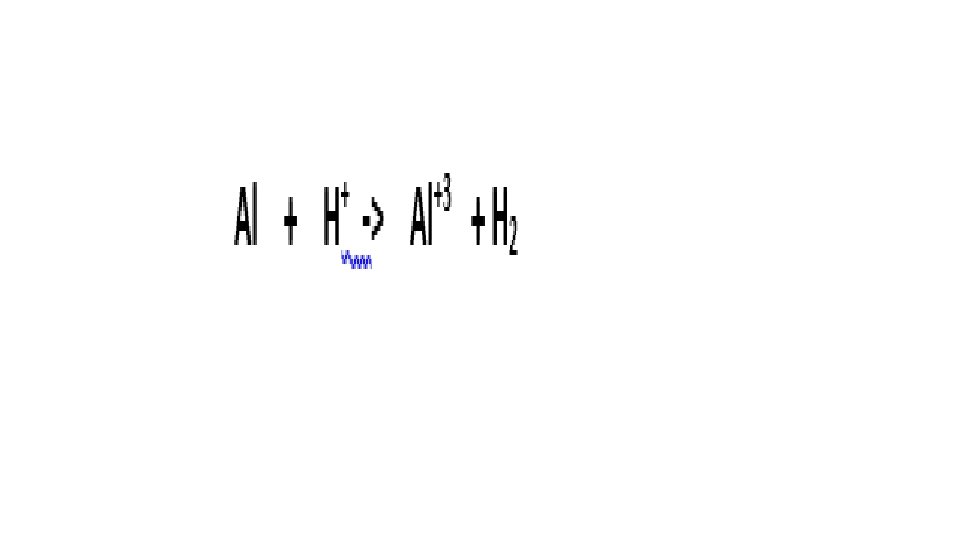
A reaction is Redox if…Oxidation number changes for 2 elements within a reaction Reduction(GER)= gain of e- by an atom or ion; oxidation # goes down/reduces Oxidation(LEO)= loss of e- by an atom or ion; oxidation # goes up/increases

DOES THE OXIDIZING DOES THE REDUCING SPECIES REDUCED OXIDIZED REACTANT SIDE



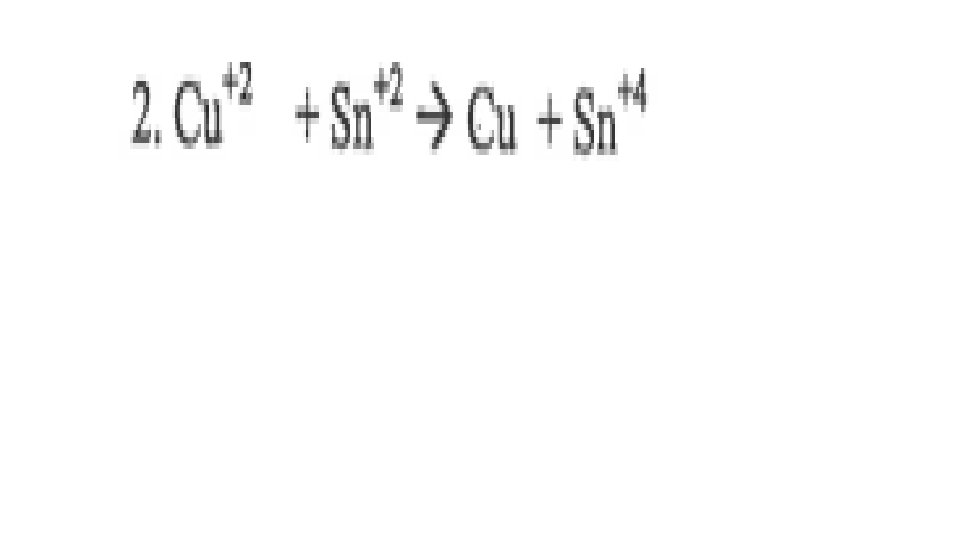
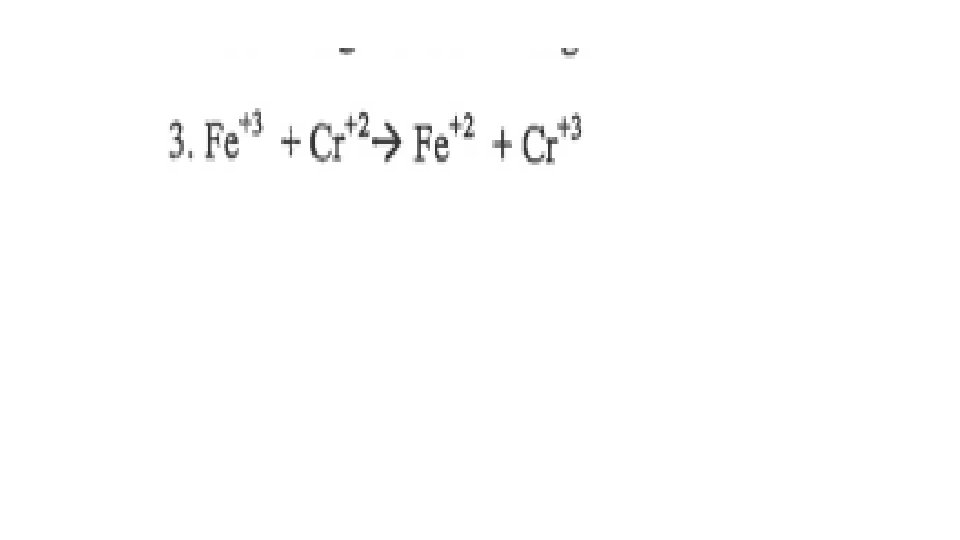

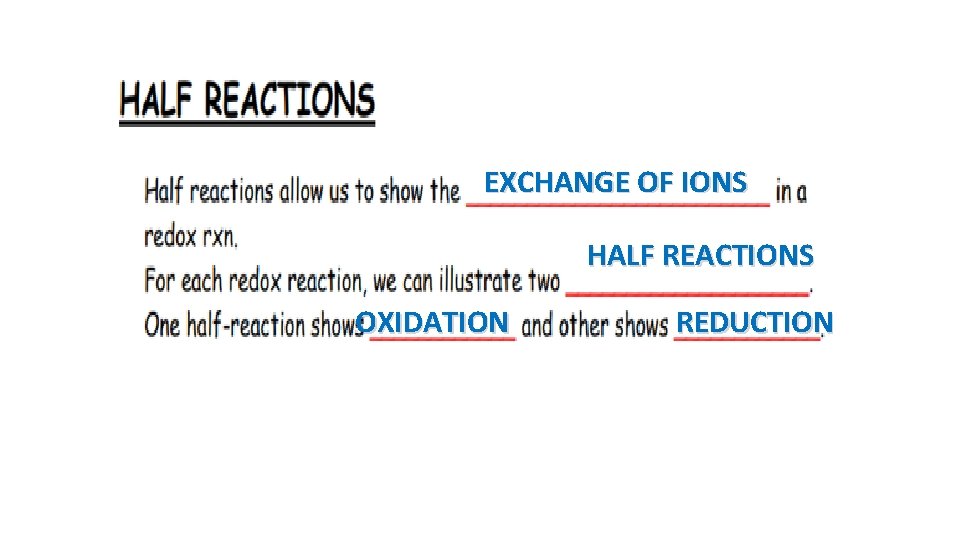
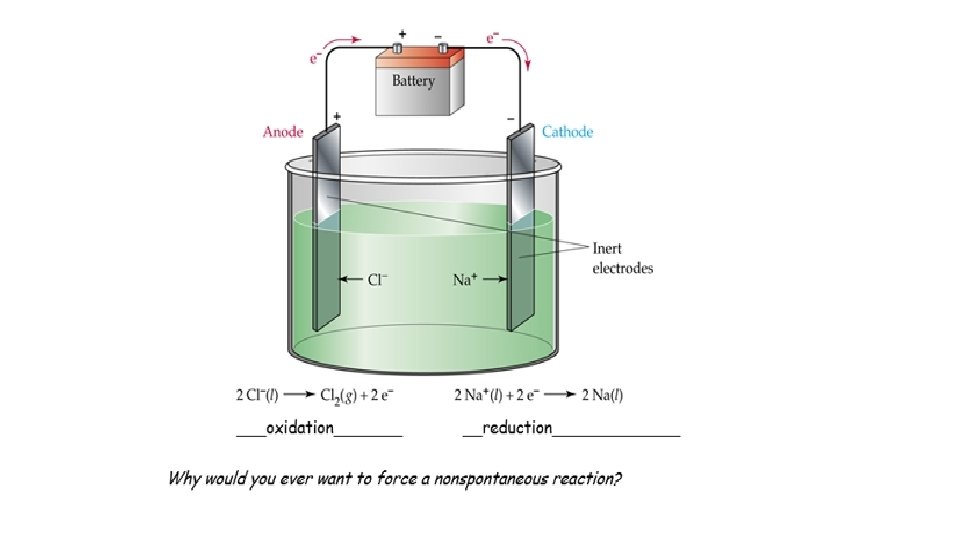
EXCHANGE OF IONS HALF REACTIONS OXIDATION REDUCTION

GAINED REDUCTION GER GAINED GER

LOST OXIDATION LEO ADD e. HIGHER TOTAL CHARGE LEO LOST NEGATIVE

LAW OF CONSERVATION OF MASS SAME # OF ATOMS CONSERVATION OF CHARGE NET CHARGE MUST BE THE SAME ON BOTH SIDES



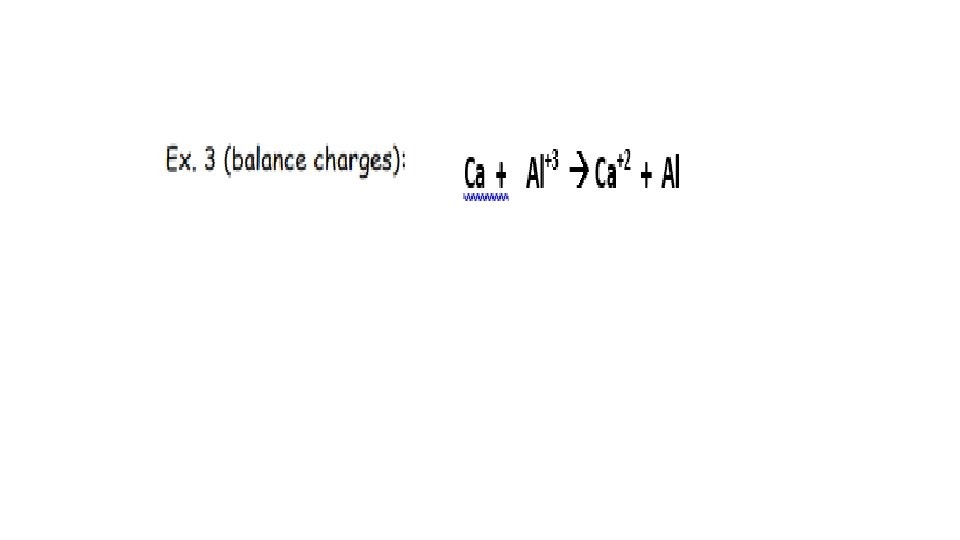


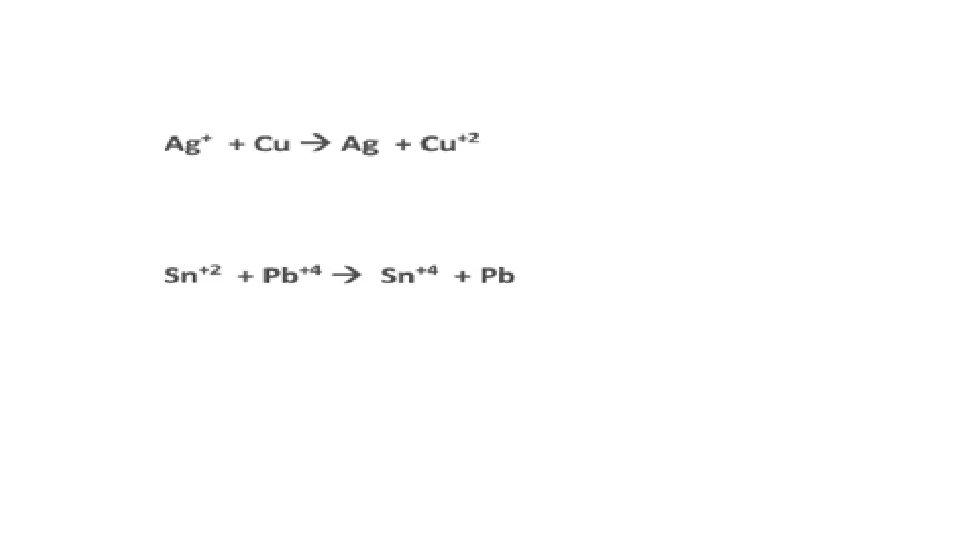


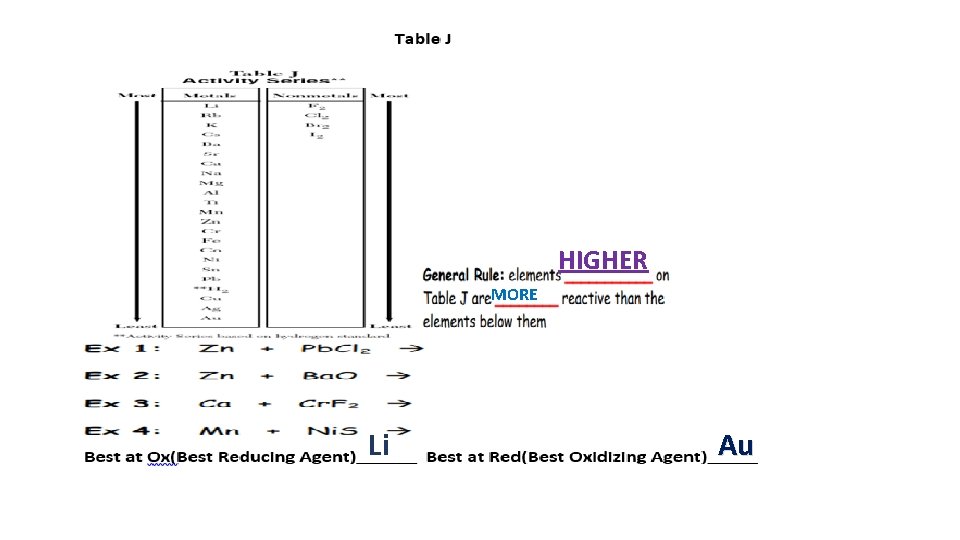
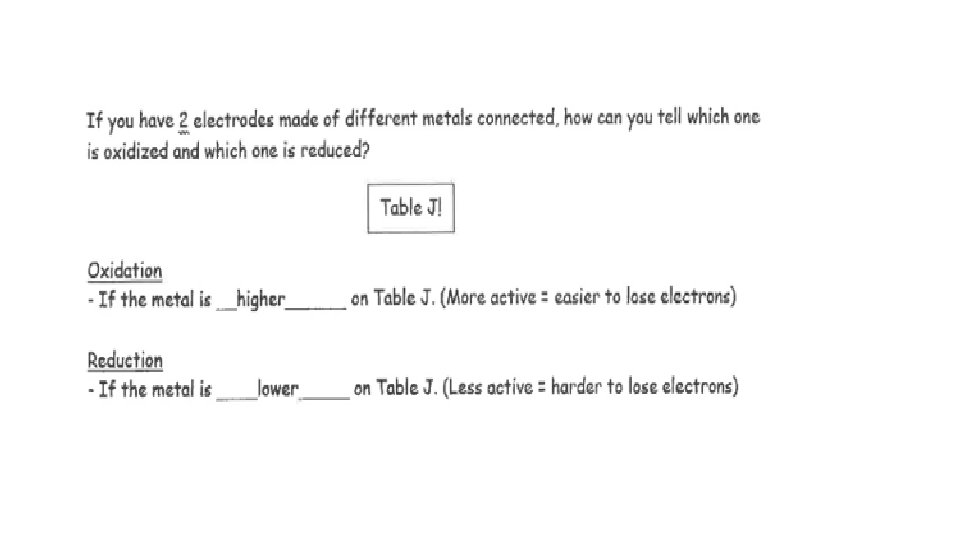
HIGHER MORE Li Au

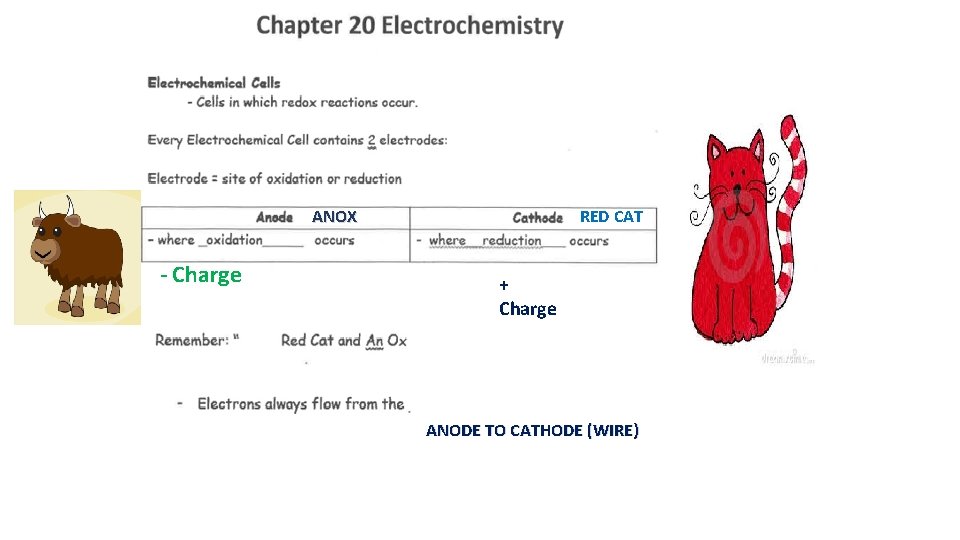
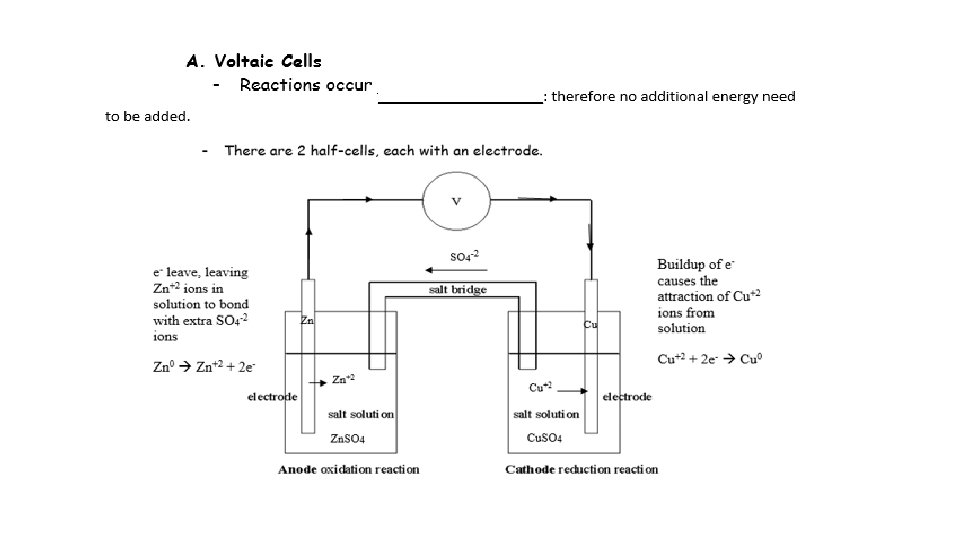
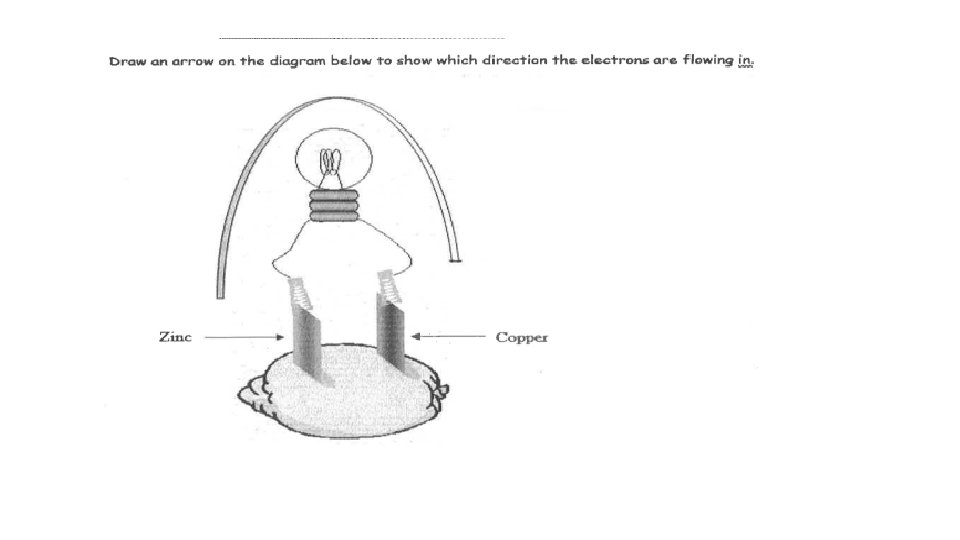
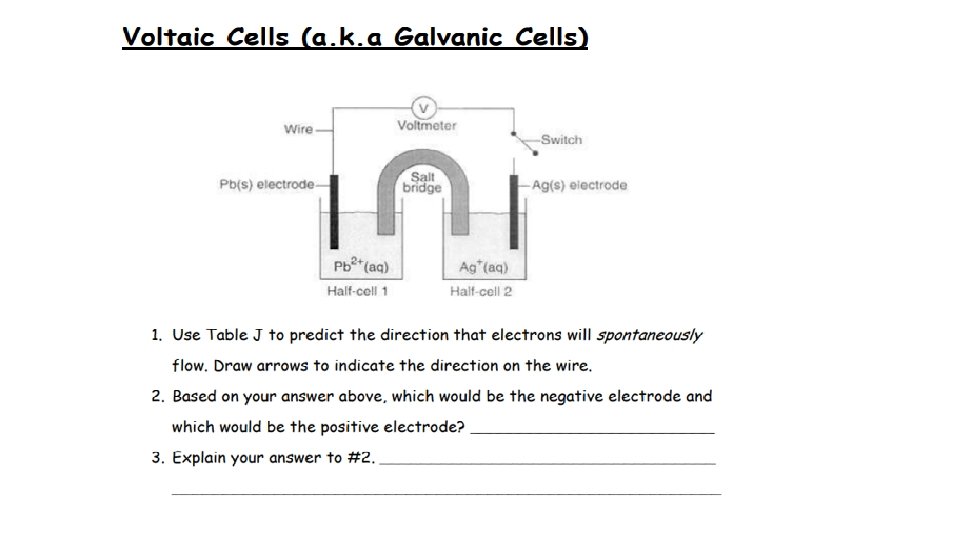
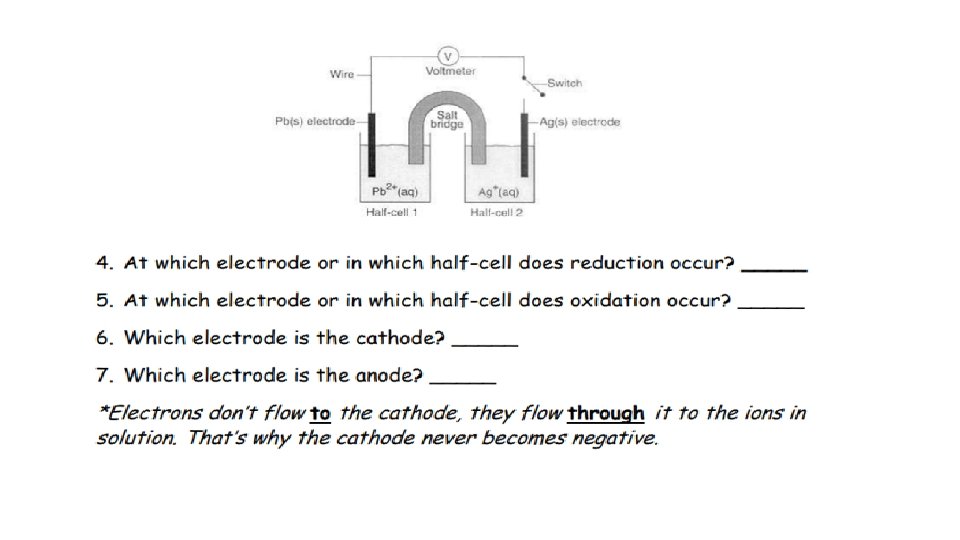
ANOX - Charge RED CAT + Charge ANODE TO CATHODE (WIRE)





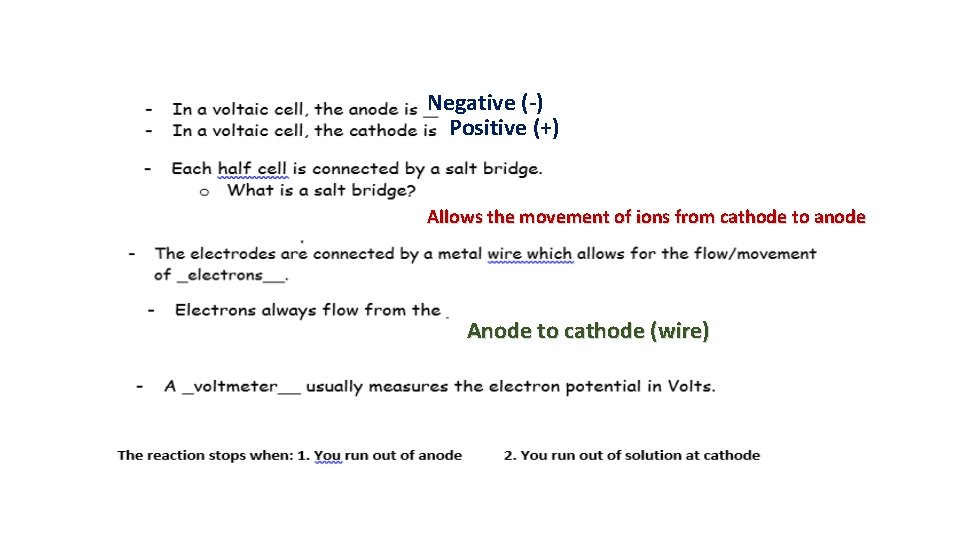
Negative (-) Positive (+) Allows the movement of ions from cathode to anode Anode to cathode (wire)





+ POSITIVE - NEGATIVE



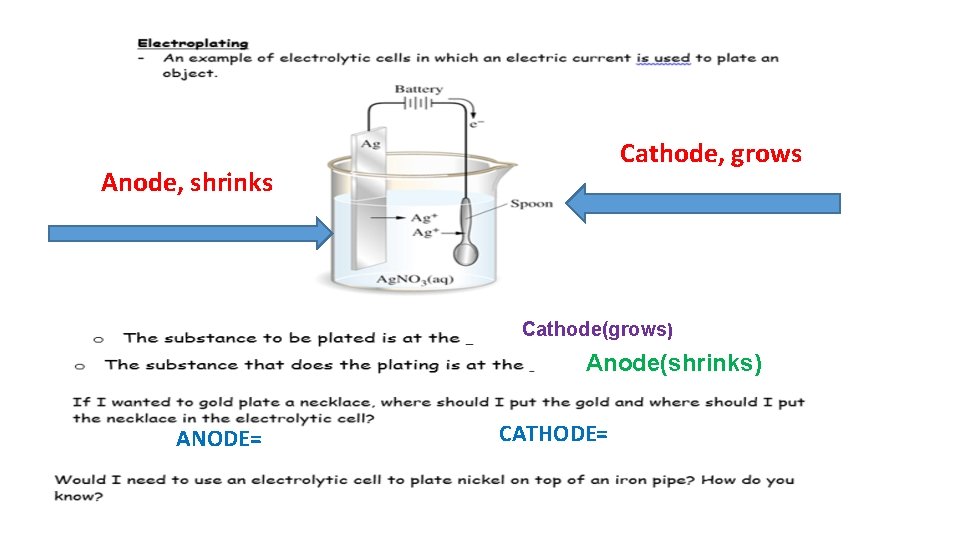
Cathode, grows Anode, shrinks Cathode(grows) Anode(shrinks) ANODE= CATHODE=