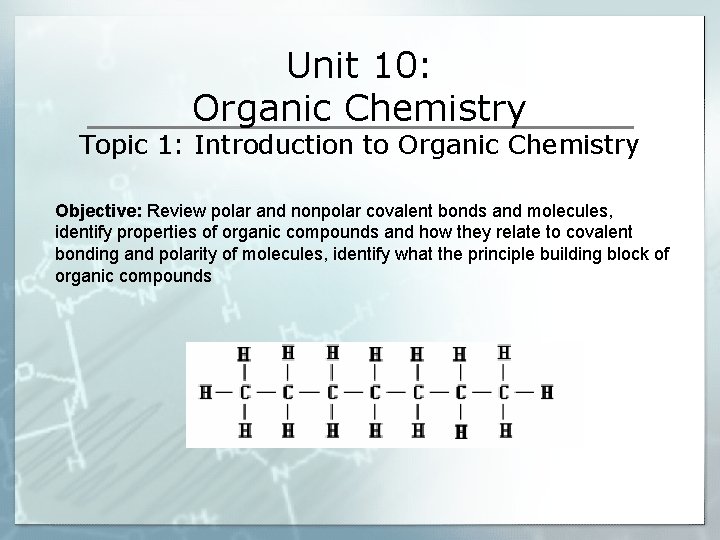
Unit 10 Organic Chemistry Topic 1 Introduction to





- Slides: 40

Unit 10: Organic Chemistry Topic 1: Introduction to Organic Chemistry Objective: Review polar and nonpolar covalent bonds and molecules, identify properties of organic compounds and how they relate to covalent bonding and polarity of molecules, identify what the principle building block of organic compounds

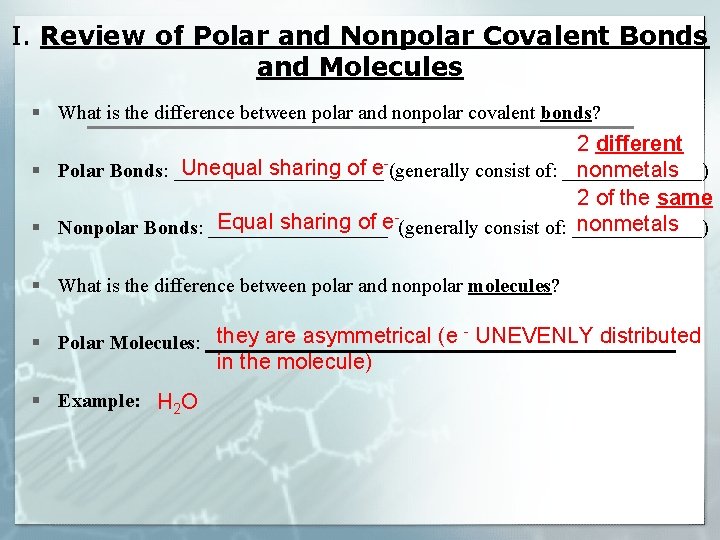
I. Review of Polar and Nonpolar Covalent Bonds and Molecules § What is the difference between polar and nonpolar covalent bonds? 2 different Unequal sharing of e§ Polar Bonds: ___________ (generally consist of: _______) nonmetals 2 of the same Equal sharing of enonmetals § Nonpolar Bonds: _________ (generally consist of: _______) § What is the difference between polar and nonpolar molecules? they are asymmetrical (e UNEVENLY distributed § Polar Molecules: ________________________ - in the molecule) § Example: H 2 O

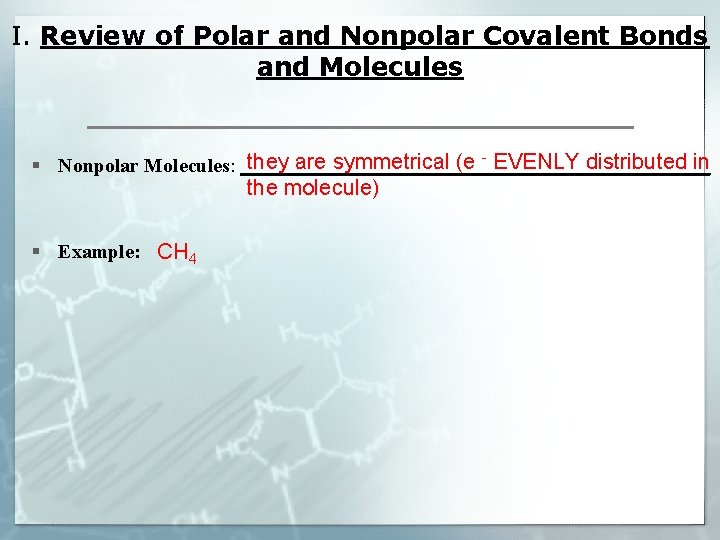
I. Review of Polar and Nonpolar Covalent Bonds and Molecules they are symmetrical (e - EVENLY distributed in § Nonpolar Molecules: ________________________ the molecule) § Example: CH 4

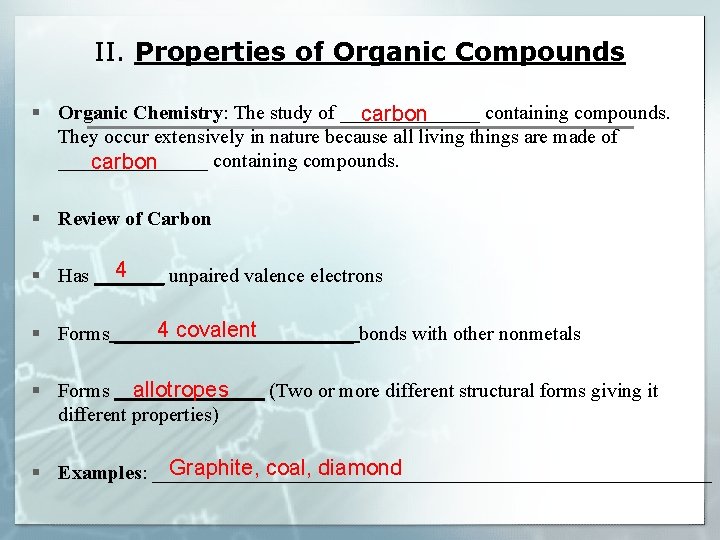
II. Properties of Organic Compounds § Organic Chemistry: The study of _______ containing compounds. carbon They occur extensively in nature because all living things are made of ________ containing compounds. carbon § Review of Carbon 4 § Has _______ unpaired valence electrons 4 covalent § Forms ____________ bonds with other nonmetals allotropes § Forms ________ (Two or more different structural forms giving it different properties) Graphite, coal, diamond § Examples: ____________________________

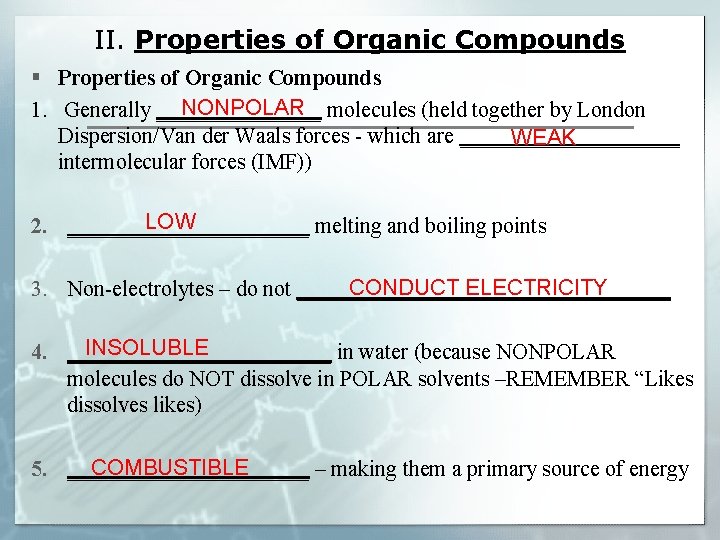
II. Properties of Organic Compounds § Properties of Organic Compounds NONPOLAR 1. Generally ________ molecules (held together by London Dispersion/Van der Waals forces - which are __________ WEAK intermolecular forces (IMF)) LOW 2. ___________ melting and boiling points CONDUCT ELECTRICITY 3. Non-electrolytes – do not _________________ INSOLUBLE 4. ____________ in water (because NONPOLAR molecules do NOT dissolve in POLAR solvents –REMEMBER “Likes dissolves likes) COMBUSTIBLE 5. ___________ – making them a primary source of energy

Unit 10: Organic Chemistry Topic 2: Naming and Writing Formulas for Organic Compounds Objective: Identify hydrocarbons, difference between saturated vs. unsaturated hydrocarbons, isomers, write the molecular formulas and structural formulas for alkanes, alkenes, and alkynes,

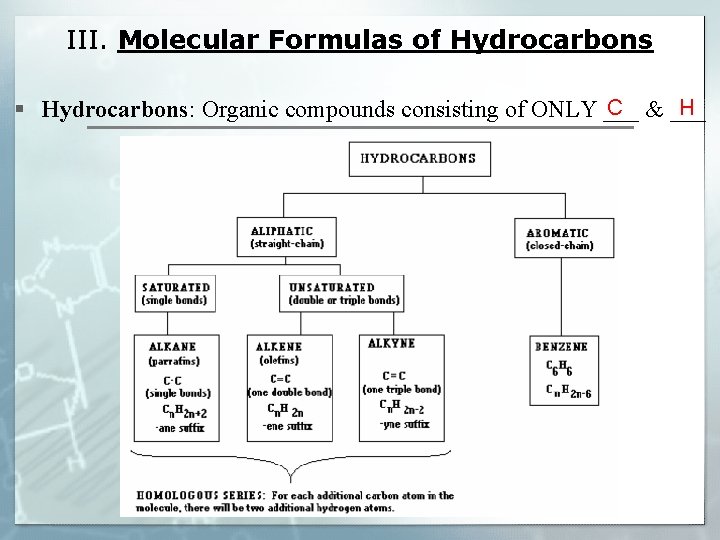
III. Molecular Formulas of Hydrocarbons C H § Hydrocarbons: Organic compounds consisting of ONLY ___ & ___

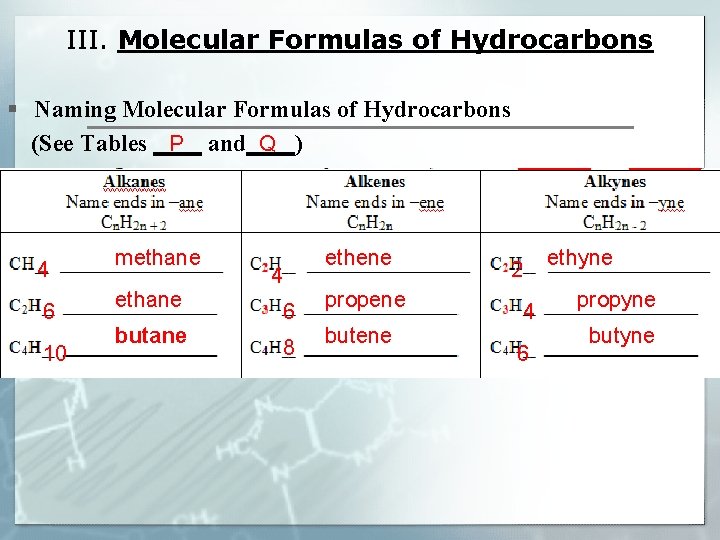
III. Molecular Formulas of Hydrocarbons § Naming Molecular Formulas of Hydrocarbons P Q (See Tables ____ and____) 4 6 10 methane butane 4 6 8 ethene propene butene 2 4 6 ethyne propyne butyne

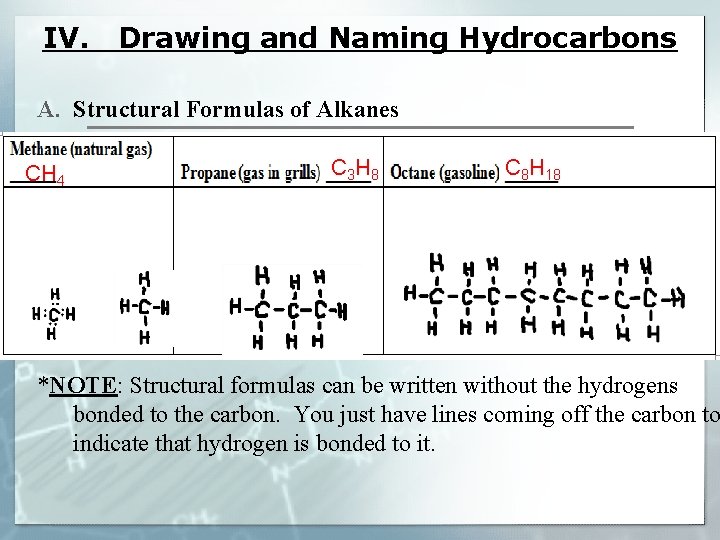
IV. Drawing and Naming Hydrocarbons A. Structural Formulas of Alkanes CH 4 C 3 H 8 C 8 H 18 *NOTE: Structural formulas can be written without the hydrogens bonded to the carbon. You just have lines coming off the carbon to indicate that hydrogen is bonded to it.

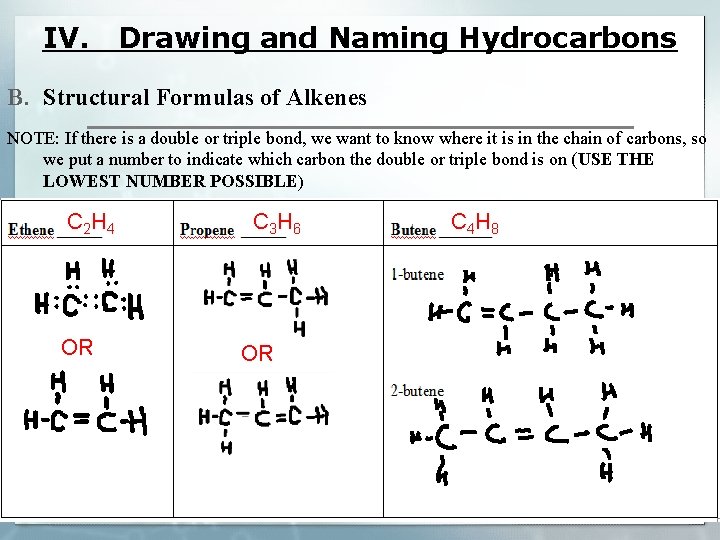
IV. Drawing and Naming Hydrocarbons B. Structural Formulas of Alkenes NOTE: If there is a double or triple bond, we want to know where it is in the chain of carbons, so we put a number to indicate which carbon the double or triple bond is on (USE THE LOWEST NUMBER POSSIBLE) C 2 H 4 OR C 3 H 6 OR C 4 H 8

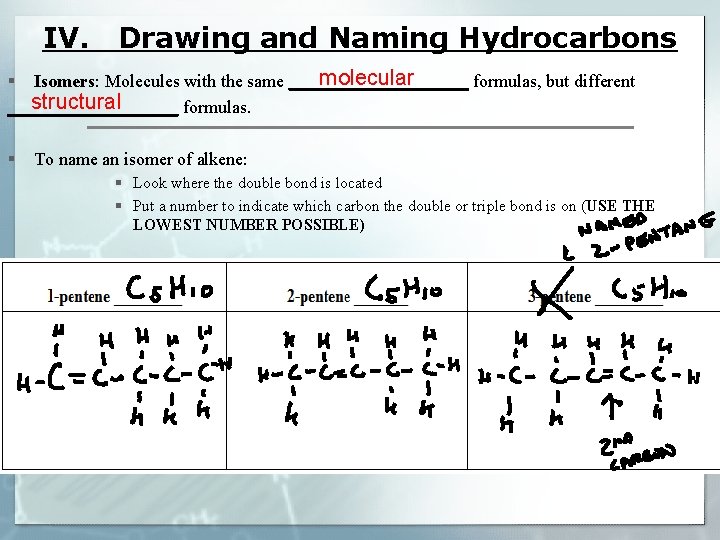
IV. Drawing and Naming Hydrocarbons molecular Isomers: Molecules with the same __________ formulas, but different § structural __________ formulas. § To name an isomer of alkene: § Look where the double bond is located § Put a number to indicate which carbon the double or triple bond is on (USE THE LOWEST NUMBER POSSIBLE)

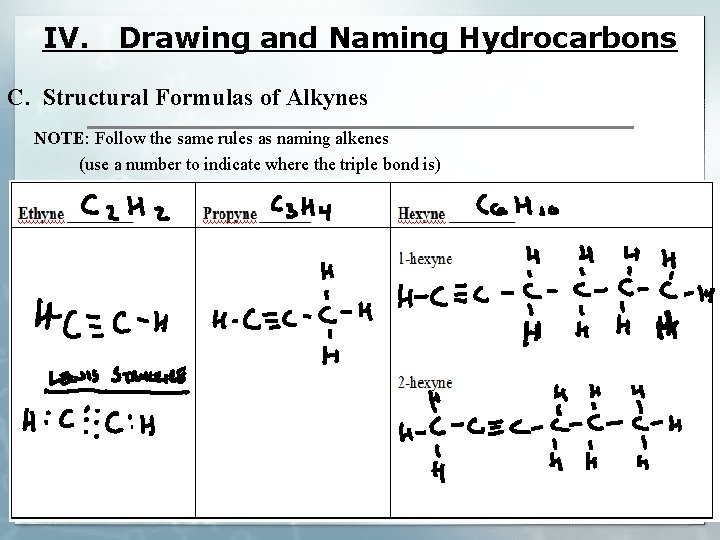
IV. Drawing and Naming Hydrocarbons C. Structural Formulas of Alkynes NOTE: Follow the same rules as naming alkenes (use a number to indicate where the triple bond is)

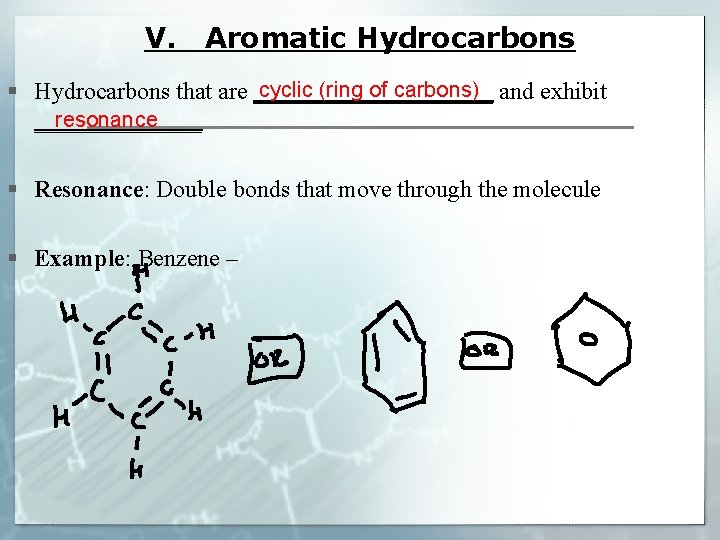
V. Aromatic Hydrocarbons cyclic (ring of carbons) § Hydrocarbons that are __________ and exhibit resonance _______ § Resonance: Double bonds that move through the molecule § Example: Benzene –

VI. Network Structures of Hydrocarbons § Carbon can make different network structures (these are allotropes of carbon) § Example: Graphite § Other Examples: nanotubes, diamonds, bucky balls

VII. Saturated vs. Unsaturated Hydrocarbons ALL SINGLE BONDS § Saturated Hydrocarbons: hydrocarbons with _________ ALKANES § Family Name: __________ § Example: Butane

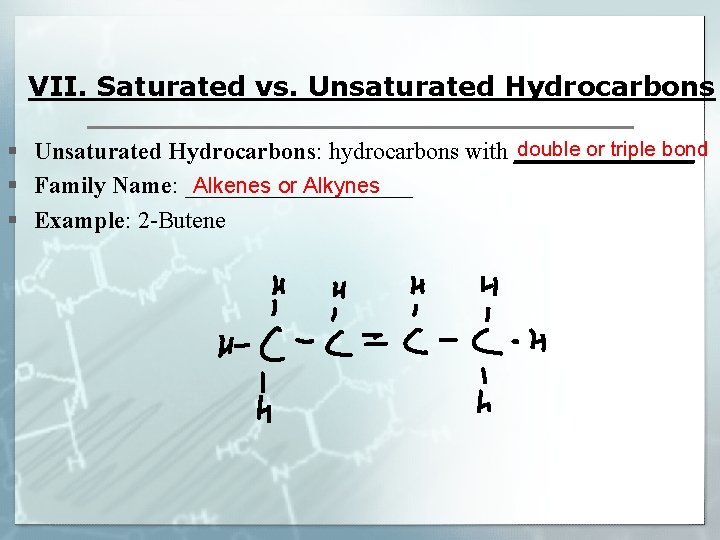
VII. Saturated vs. Unsaturated Hydrocarbons double or triple bond § Unsaturated Hydrocarbons: hydrocarbons with ________ Alkenes or Alkynes § Family Name: __________ § Example: 2 -Butene

Unit 10: Organic Chemistry Topic 3: Branched (Substituted) Hydrocarbons Objective: Draw structural formulas of branched hydrocarbons from their names, write the names of branched hydrocarbons from their structural formulas, identify isomers of branched hydrocarbons

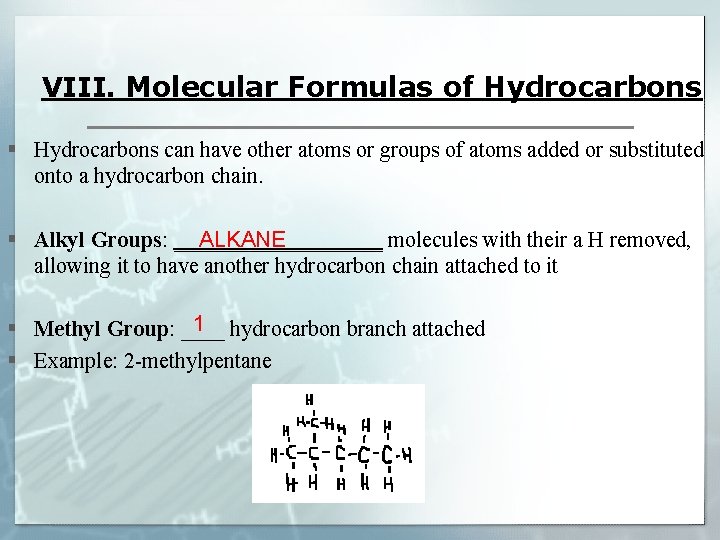
VIII. Molecular Formulas of Hydrocarbons § Hydrocarbons can have other atoms or groups of atoms added or substituted onto a hydrocarbon chain. § Alkyl Groups: __________ molecules with their a H removed, ALKANE allowing it to have another hydrocarbon chain attached to it 1 § Methyl Group: ____ hydrocarbon branch attached § Example: 2 -methylpentane

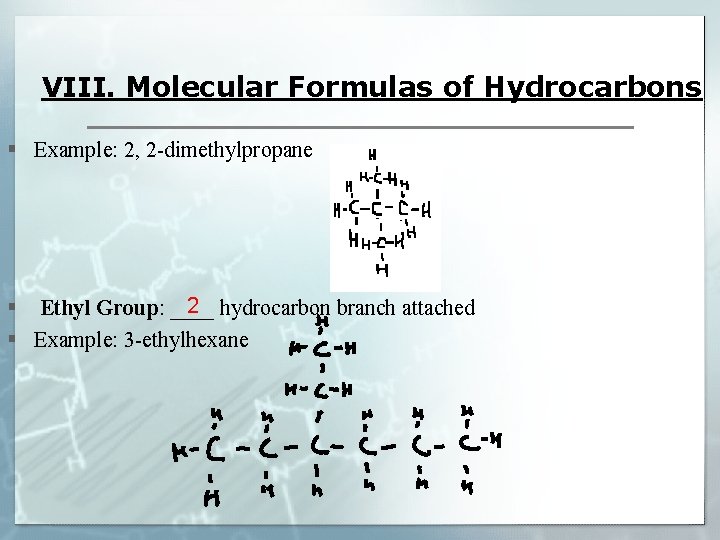
VIII. Molecular Formulas of Hydrocarbons § Example: 2, 2 -dimethylpropane 2 § Ethyl Group: ____ hydrocarbon branch attached § Example: 3 -ethylhexane

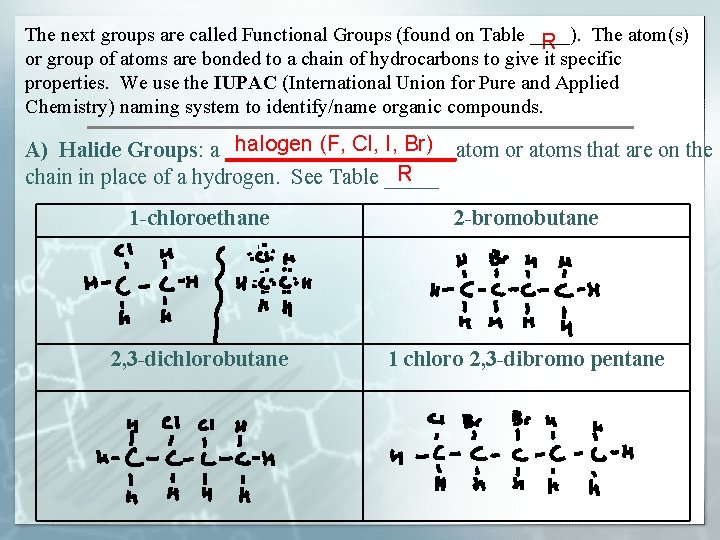
The next groups are called Functional Groups (found on Table ____). The atom(s) R or group of atoms are bonded to a chain of hydrocarbons to give it specific properties. We use the IUPAC (International Union for Pure and Applied Chemistry) naming system to identify/name organic compounds. halogen (F, Cl, I, Br) A) Halide Groups: a ___________atom or atoms that are on the R chain in place of a hydrogen. See Table _____ 1 -chloroethane 2 -bromobutane 2, 3 -dichlorobutane 1 chloro 2, 3 -dibromo pentane

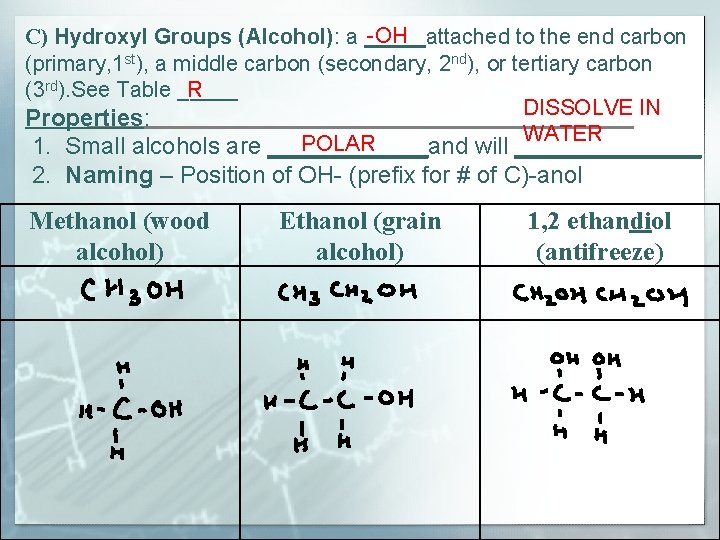
-OH C) Hydroxyl Groups (Alcohol): a _____attached to the end carbon (primary, 1 st), a middle carbon (secondary, 2 nd), or tertiary carbon R (3 rd). See Table _____ DISSOLVE IN Properties: WATER POLAR 1. Small alcohols are ______and will _______ 2. Naming – Position of OH- (prefix for # of C)-anol Methanol (wood alcohol) Ethanol (grain alcohol) 1, 2 ethandiol (antifreeze)

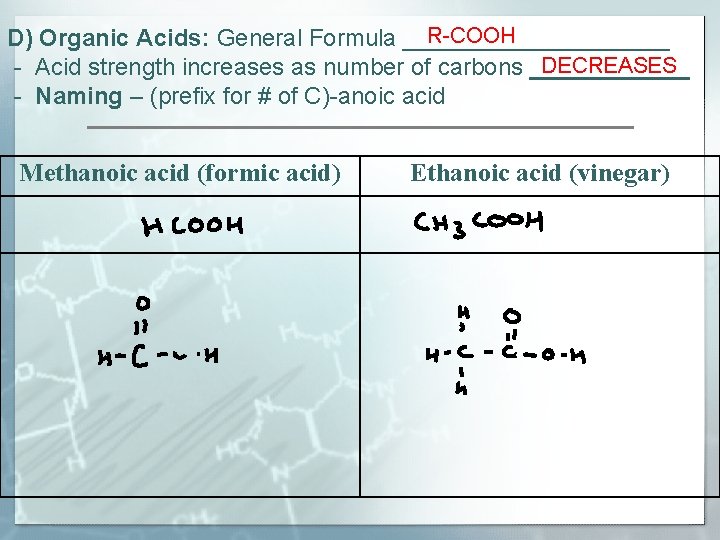
R-COOH D) Organic Acids: General Formula __________ DECREASES - Acid strength increases as number of carbons ______ - Naming – (prefix for # of C)-anoic acid Methanoic acid (formic acid) Ethanoic acid (vinegar)

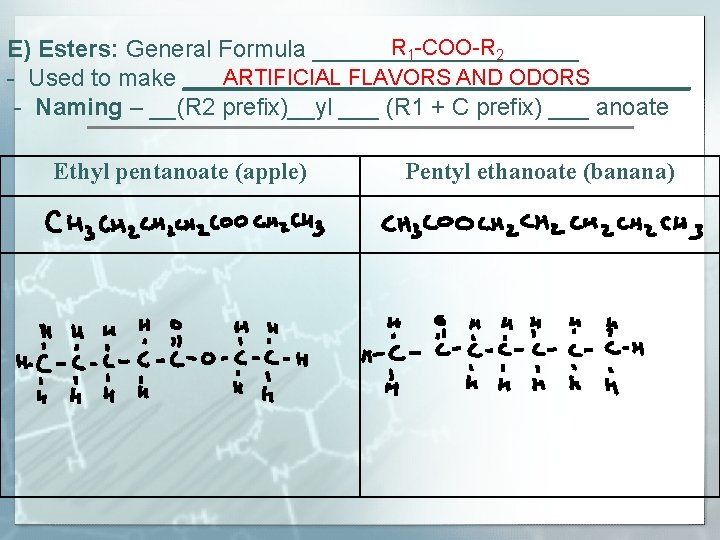
R 1 -COO-R 2 E) Esters: General Formula __________ ARTIFICIAL FLAVORS AND ODORS - Used to make ___________________ - Naming – __(R 2 prefix)__yl ___ (R 1 + C prefix) ___ anoate Ethyl pentanoate (apple) Pentyl ethanoate (banana)

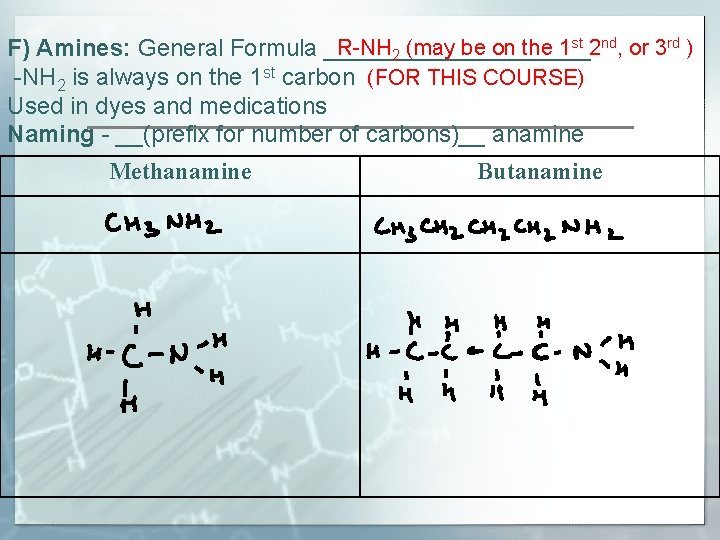
R-NH 2 (may be on the 1 st 2 nd, or 3 rd ) F) Amines: General Formula __________ -NH 2 is always on the 1 st carbon (FOR THIS COURSE) Used in dyes and medications Naming - __(prefix for number of carbons)__ anamine Methanamine Butanamine

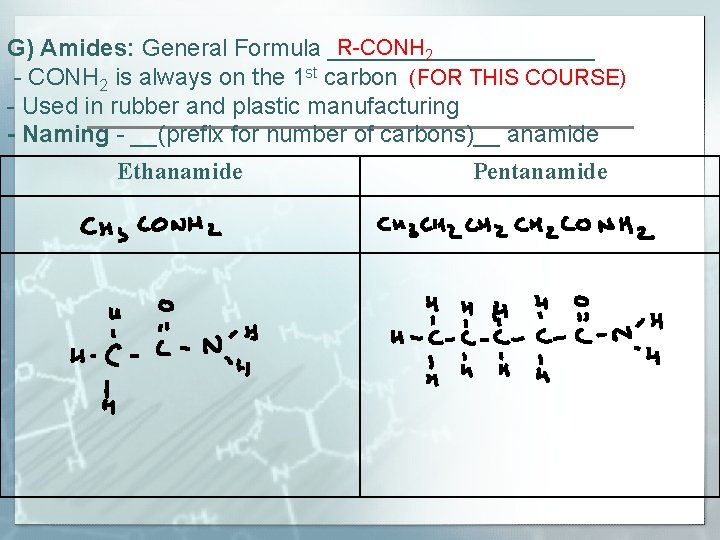
R-CONH 2 G) Amides: General Formula __________ - CONH 2 is always on the 1 st carbon (FOR THIS COURSE) - Used in rubber and plastic manufacturing - Naming - __(prefix for number of carbons)__ anamide Ethanamide Pentanamide

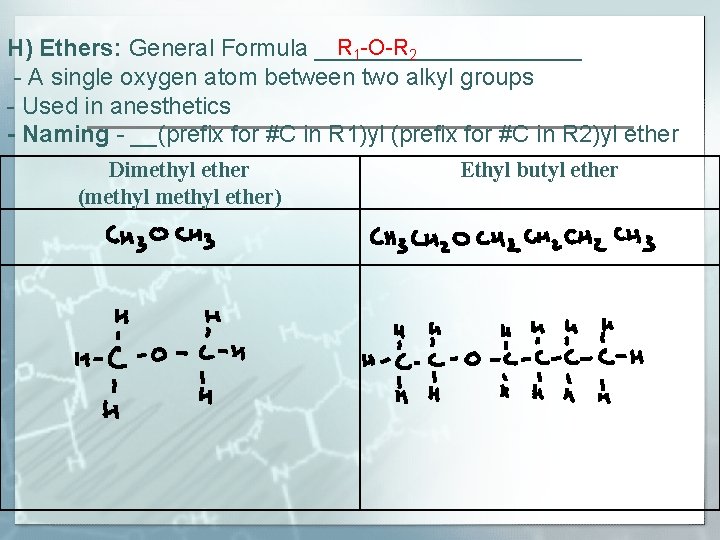
R 1 -O-R 2 H) Ethers: General Formula __________ - A single oxygen atom between two alkyl groups - Used in anesthetics - Naming - __(prefix for #C in R 1)yl (prefix for #C in R 2)yl ether Dimethyl ether (methyl ether) Ethyl butyl ether

R-CHO I) Aldehyde: General Formula __________ -COH is on the first carbon - Used in preservatives - Naming - __(prefix for #C)-anal Methanal Ethanal

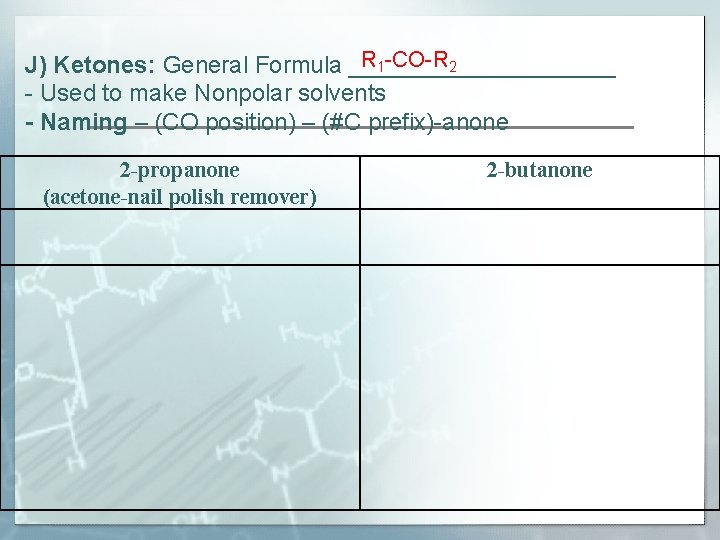
R 1 -CO-R 2 J) Ketones: General Formula __________ - Used to make Nonpolar solvents - Naming – (CO position) – (#C prefix)-anone 2 -propanone (acetone-nail polish remover) 2 -butanone

Unit 10: Organic Chemistry Topic 4: Organic Reactions Objective: Determine what kind of reaction is required to make the desired organic product, complete simple organic reactions, and identify the reaction that is proceeding based on structural formulas or molecular formulas

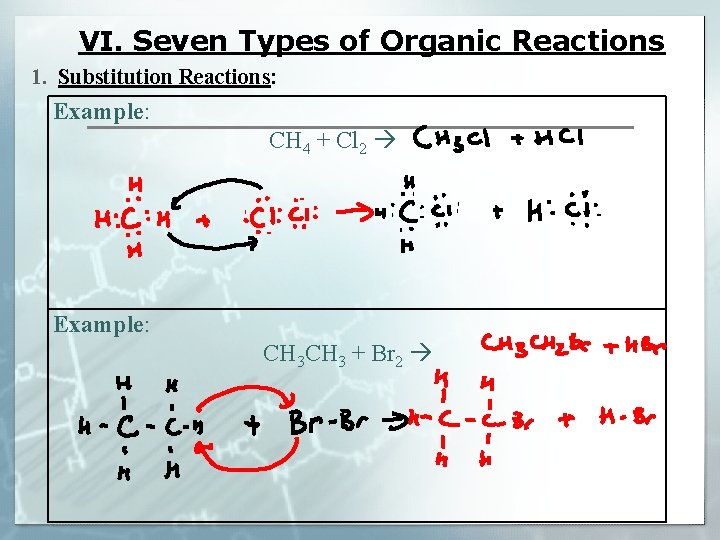
VI. Seven Types of Organic Reactions § In general, these are slower than inorganic reactions because bonds need to be broken § Frequently involve only the functional groups of the molecule, the majority of the molecule remains unchanged 1. Substitution Reactions: SATURATED ALKANES § Involve __________ hydrocarbons (__________) HYDROGENS § One of the ___________ are removed (usually on the end) HALOGEN § It is replaced with an atom of __________. HA is the byproduct

VI. Seven Types of Organic Reactions 1. Substitution Reactions: Example: CH 4 + Cl 2 Example: CH 3 + Br 2

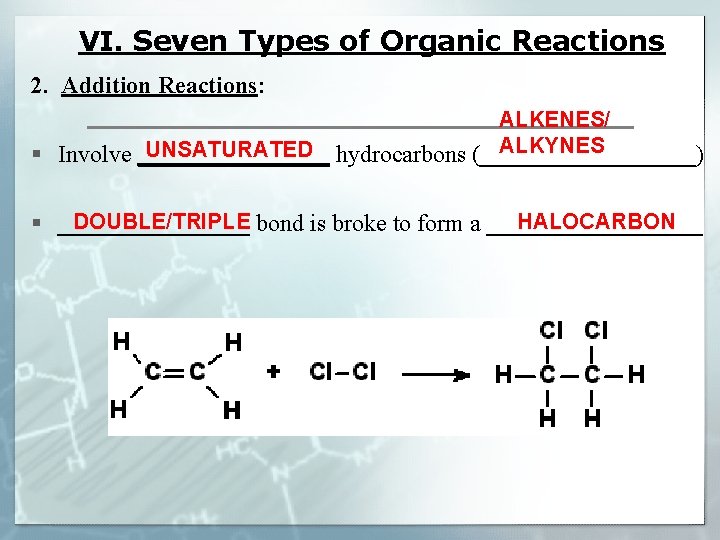
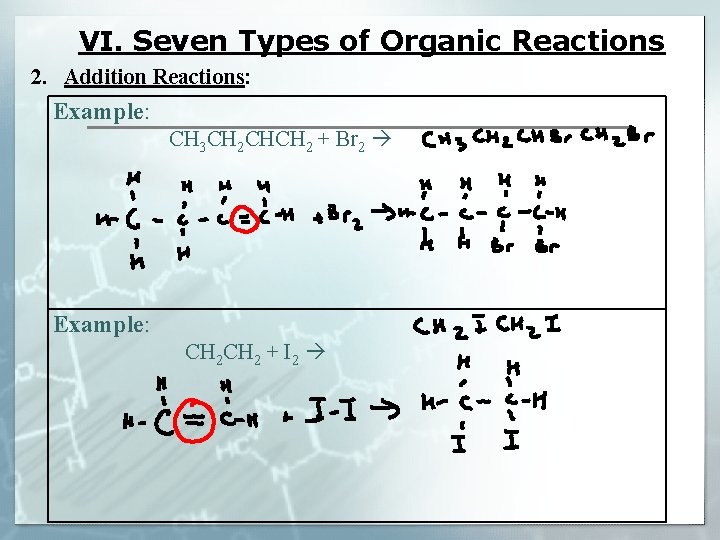
VI. Seven Types of Organic Reactions 2. Addition Reactions: ALKENES/ ALKYNES UNSATURATED § Involve ________ hydrocarbons (_________) DOUBLE/TRIPLE HALOCARBON § ________ bond is broke to form a _________

VI. Seven Types of Organic Reactions 2. Addition Reactions: Example: CH 3 CH 2 CHCH 2 + Br 2 Example: CH 2 + I 2

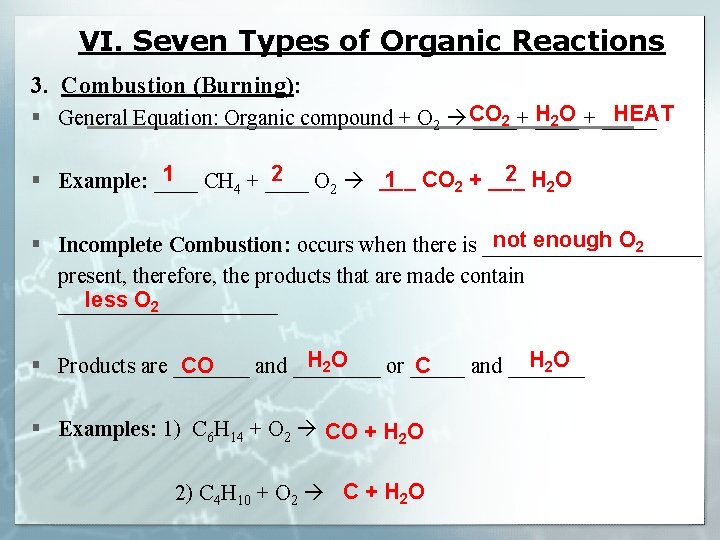
VI. Seven Types of Organic Reactions 3. Combustion (Burning): CO 2 H 2 O HEAT § General Equation: Organic compound + O 2 ____+ _____ 1 2 2 HO 1 CO 2 + ___ § Example: ____ CH 2 4 + ____ O 2 ___ not enough O 2 § Incomplete Combustion: occurs when there is __________ present, therefore, the products that are made contain less O 2 __________ H 2 O CO C § Products are _______ and ____ or _____ and _______ § Examples: 1) C 6 H 14 + O 2 CO + H 2 O 2) C 4 H 10 + O 2 C + H 2 O

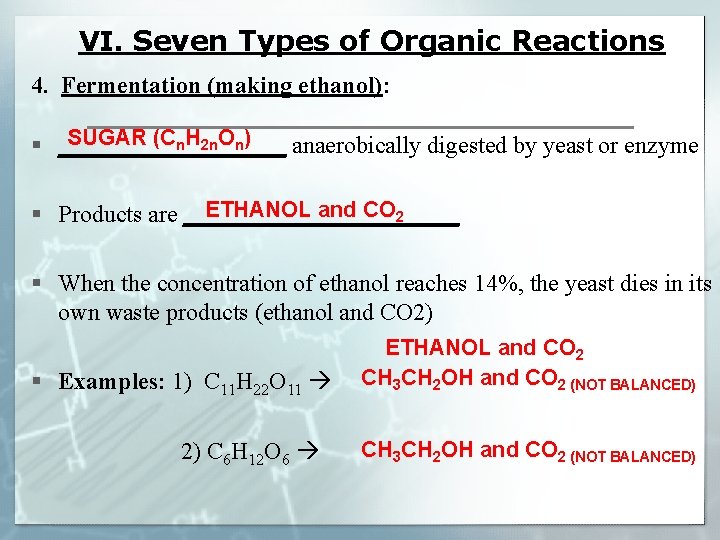
VI. Seven Types of Organic Reactions 4. Fermentation (making ethanol): SUGAR (Cn. H 2 n. On) § __________ anaerobically digested by yeast or enzyme ETHANOL and CO 2 § Products are ____________ § When the concentration of ethanol reaches 14%, the yeast dies in its own waste products (ethanol and CO 2) § Examples: 1) C 11 H 22 O 11 2) C 6 H 12 O 6 ETHANOL and CO 2 CH 3 CH 2 OH and CO 2 (NOT BALANCED)

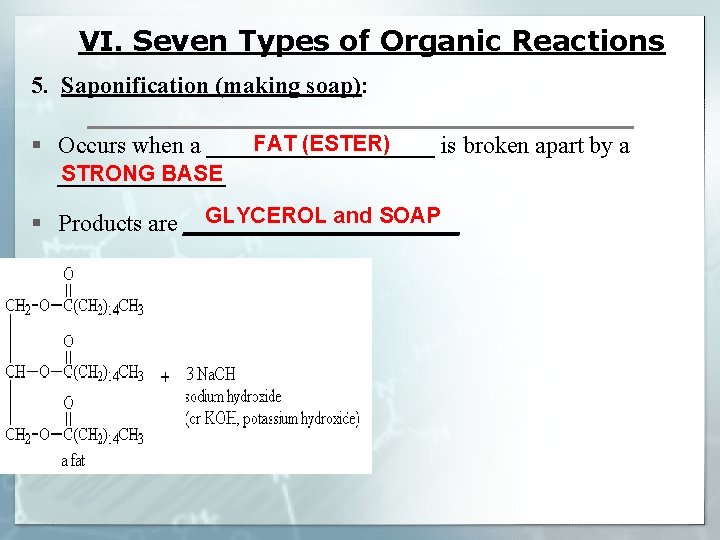
VI. Seven Types of Organic Reactions 5. Saponification (making soap): FAT (ESTER) § Occurs when a __________ is broken apart by a STRONG BASE _______ GLYCEROL and SOAP § Products are ____________

VI. Seven Types of Organic Reactions 6. Esterification (making esters): ORGANIC ACID ALCOHOL § Occur when an ________ reacts with an _______ DEHYDRATION SYNTHESIS (WATER REMOVED) § Occurs by ______________________ ESTER H 2 O § Products are ______ and ________ § Example: Methanoic acid + ethanol Water + ethyl methanoate

VI. Seven Types of Organic Reactions 7. Polymerization (making polymers): Intro - VIDEO ANY ORGANIC SUBSTANCE with REPEATING UNITS § Polymer: _______________________ BASIC ORGANIC UNIT OF ANY POLYMER § Monomer: ______________________ RUBBER and PLASTICS § Synthetic Examples: ___________________ PROTEINS, STARCH, and DNA § Natural Examples: ____________________

VI. Seven Types of Organic Reactions A. Addition Polymerization UNSATURATED (DOUBLE/TRIPLE BONDED) § Occurs with a _________________ hydrocarbon BROKEN AND USED TO BOND § The double or triple bond is… _______________ THE MONOMERS TOGETHER IN A LONG CHAIN ____________________________ POLYMER § The product is a ___________ § Example: vinyl chloride polyvinyl chloride

VI. Seven Types of Organic Reactions B. Condensation Polymerization DEHYDRATION SYNTHESIS § Occurs by ___________________ and WATER with a POLYMER always produces _______________ § Example: