Unit 10 Chemical Reactions 10 2 Balancing chemical

Unit 10: Chemical Reactions 10. 2 Balancing chemical equations

After today you will be able to… • Explain the Law of Conservation of Atoms • Balance equations using the “tally method” • Write the out the formula equations fromheatword equations • Balance the corresponding formula Pt equation

Law of Conservation of Atoms: There must be the same number of each type of atom before the reaction as after the reaction.

Coefficients: Are numbers that go in front of each substance to indicate the number of atoms or molecules that are reacting or being produced.

Let’s try some examples! We will be using the “tally method” to balance equations in this class.
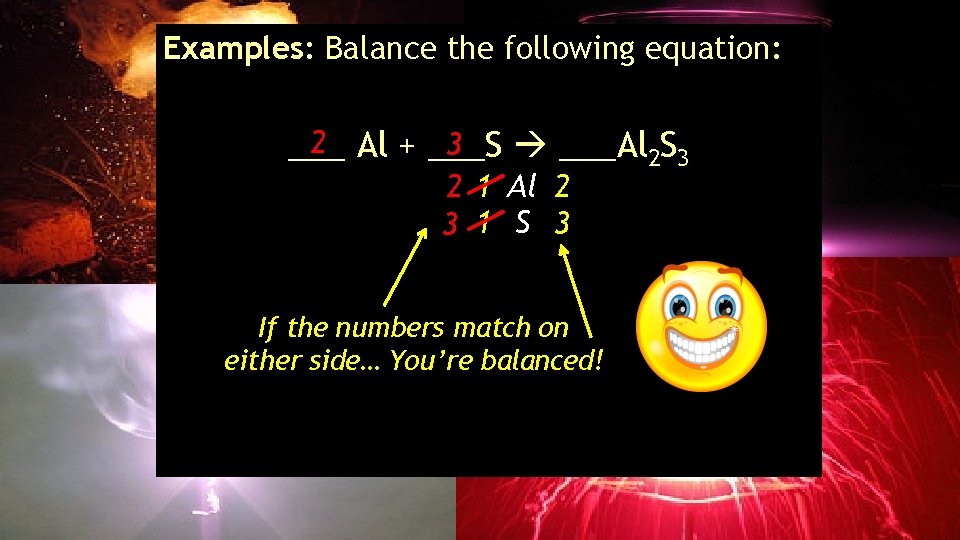
Examples: Balance the following equation: 2 Al + ___S 3 ___Al 2 S 3 2 1 Al 2 3 1 S 3 If the numbers match on either side… You’re balanced!

Examples: Balance the following equation: 42 Li + ___O ___Li 2 ___ 2 2 O 4 2 1 Li 2 4 2 O 1 2

Examples: Balance the following equation: 3 2 2 ___Fe 2(SO 4)3 + ___Na 3(PO 4) ___Fe(PO 4) + ___Na 2(SO 4) 2 Fe 1 2 3 (SO 4) 1 3 6 3 Na 2 6 2 1 (PO 4) 1 2 Helpful tip: Do not separate Are we balanced? YES!!!! poyatomic ions!

Time to put it all together!
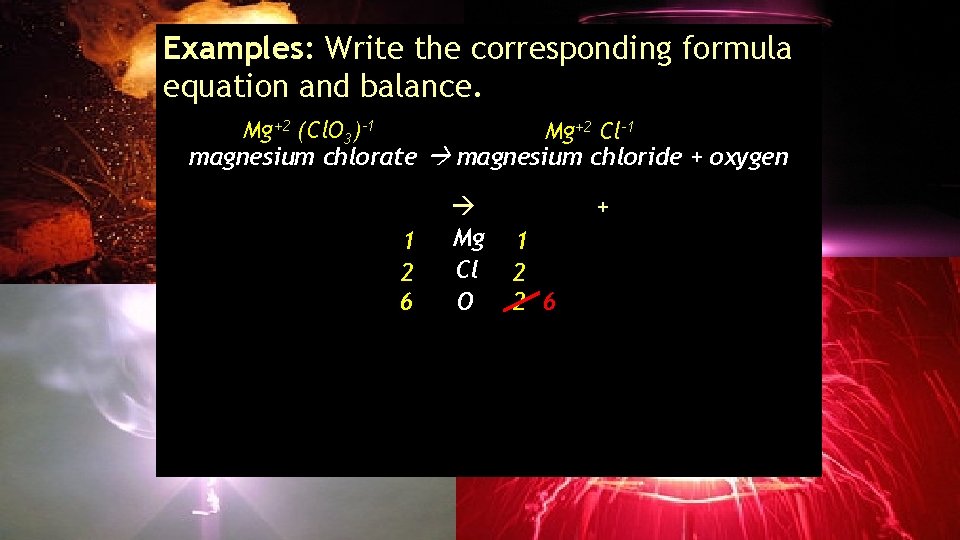
Examples: Write the corresponding formula equation and balance. Mg+2 (Cl. O 3)-1 Mg+2 Cl-1 magnesium chlorate magnesium chloride + oxygen ___Mg(Cl. O 3)2 1 2 6 3 2 ___Mg. Cl 2 + ___O Mg 1 Cl 2 O 2 6
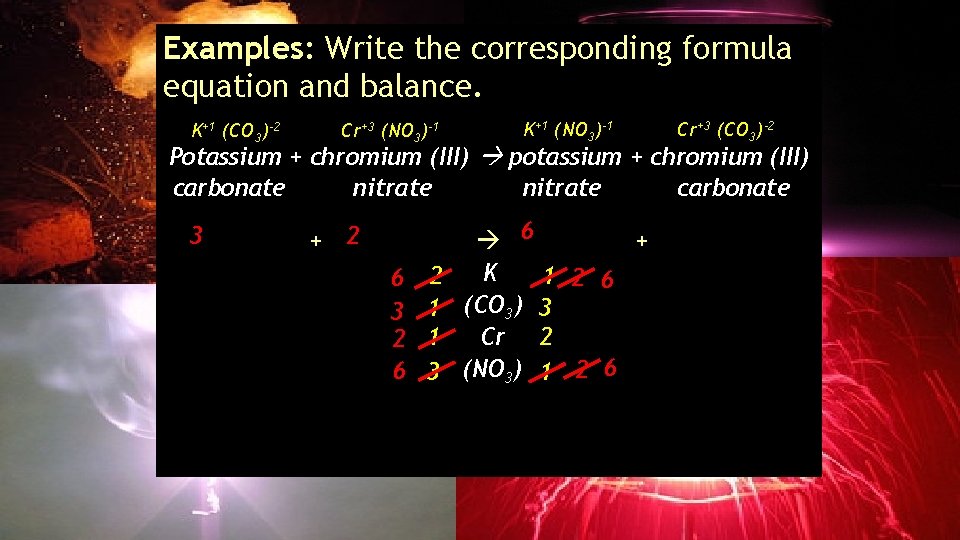
Examples: Write the corresponding formula equation and balance. K+1 (CO 3)-2 Cr+3 (NO 3)-1 K+1 (NO 3)-1 Cr+3 (CO 3)-2 Potassium + chromium (III) potassium + chromium (III) carbonate nitrate carbonate 6 3 2(CO 3) + ___Cr(NO 2 2 ___K ) ___K(NO 3 3 3) + ___Cr 2(CO 3)3 K 1 2 6 6 2 3 1 (CO 3) 3 Cr 2 2 1 6 3 (NO 3) 1 2 6

Then: Begin WS 2
- Slides: 12