Unit 10 Chemical Bonding Section 1 Ionic and
Unit 10: Chemical Bonding Section 1: Ionic and Covalent Bonding
Chemical Bonds • Forces that hold atoms or ions together to make all molecules and compounds • There are two main type of bonds – Ionic bonds and covalent bonds
Ionic Bonds • Bonds achieved by transferring valence electrons – Due to a positive (metal) and negative (nonmetal) attraction
The Lewis Symbol • In ionic bonding, atoms gain and lose electrons to achieve a noble gas electron configuration (a stable state) • Lewis symbols use dots to represent an element’s number of valence electrons – Na has 1 valence electron Na – Cl has 7 valence electrons Cl – The order for applying dots is right, left, top, bottom, right, left, top, and bottom • The maximum dots possible is 8, and the least dots possible is 1
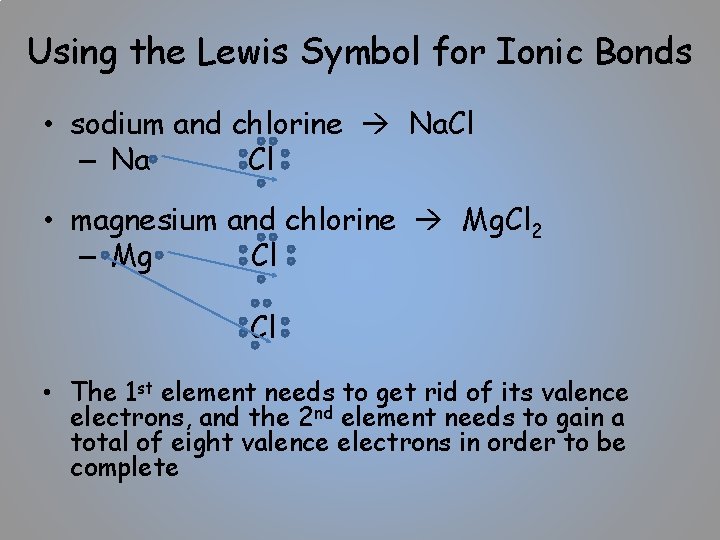
Using the Lewis Symbol for Ionic Bonds • sodium and chlorine Na. Cl – Na Cl • magnesium and chlorine Mg. Cl 2 – Mg Cl Cl • The 1 st element needs to get rid of its valence electrons, and the 2 nd element needs to gain a total of eight valence electrons in order to be complete

Covalent Bonds • Bonds achieved by sharing valence electrons – A bond between two nonmetals (two negatively charged atoms) • There are two types of covalent bonds: nonpolar covalent and polar covalent – Nonpolar covalent bonds have equally shared valence electrons, where the attraction is even throughout – Polar covalent bonds have unequally shared valence electron, where the attraction is being pulled more toward the atom with the higher electronegativity
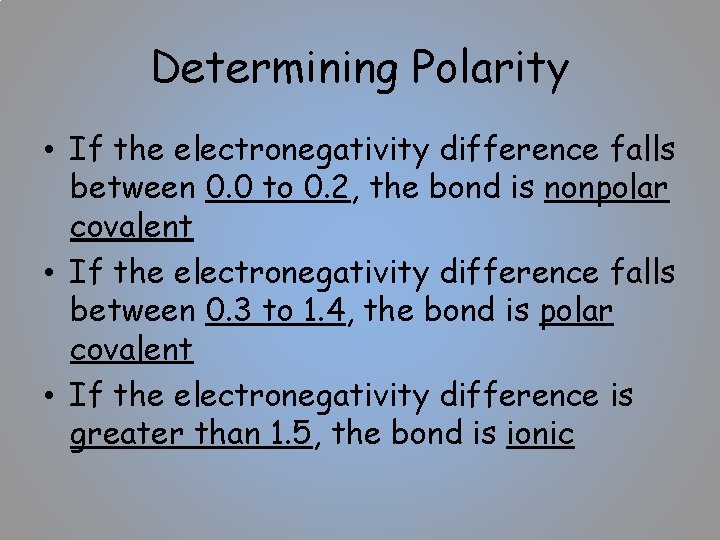
Determining Polarity • If the electronegativity difference falls between 0. 0 to 0. 2, the bond is nonpolar covalent • If the electronegativity difference falls between 0. 3 to 1. 4, the bond is polar covalent • If the electronegativity difference is greater than 1. 5, the bond is ionic
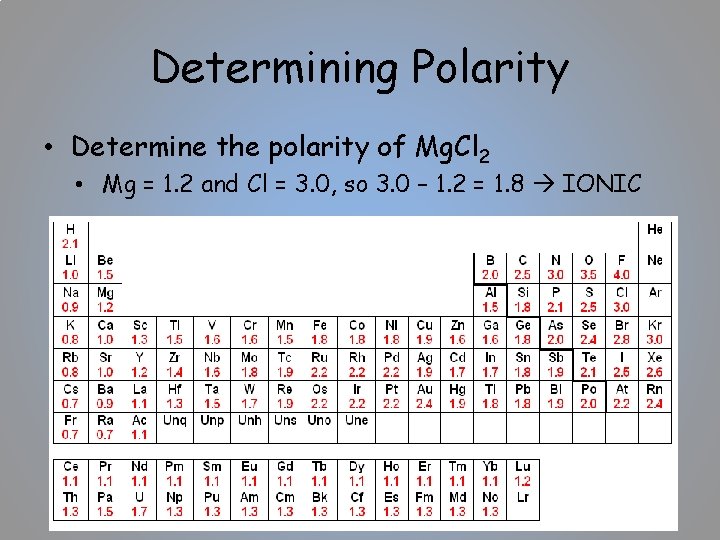
Determining Polarity • Determine the polarity of Mg. Cl 2 • Mg = 1. 2 and Cl = 3. 0, so 3. 0 – 1. 2 = 1. 8 IONIC
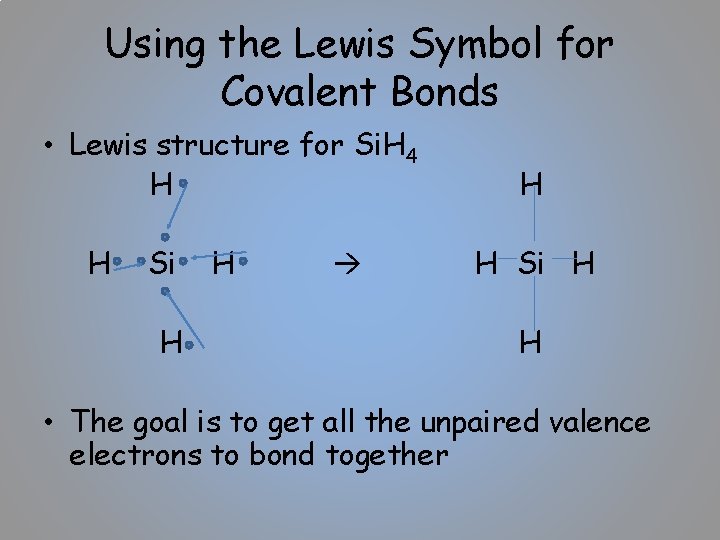
Using the Lewis Symbol for Covalent Bonds • Lewis structure for Si. H 4 H H Si H H • The goal is to get all the unpaired valence electrons to bond together
- Slides: 9