Unit 10 Chemical Bonding Nomenclature At an atomic
Unit 10 – Chemical Bonding & Nomenclature • At an atomic level, the properties and form of each atom affects the types of relationships and interactions with other atoms and compounds. 1. What are the ways in which types of bonding (ionic, covalent, metallic) differ form one another? What are specific properties of each? 2. How is electron behavior related to each type of bonding? How does it affect the properties? 3. Which elements form which types of bonding? 4. What are the rules of nomenclature for ionic and covalent bonding?

Chemical Bonds • Chemical Bond - an attractive force that compound holds atoms together in a ______ • Three types of bonding can occur – Ionic Bonding – Covalent Bonding – Metallic Bonding
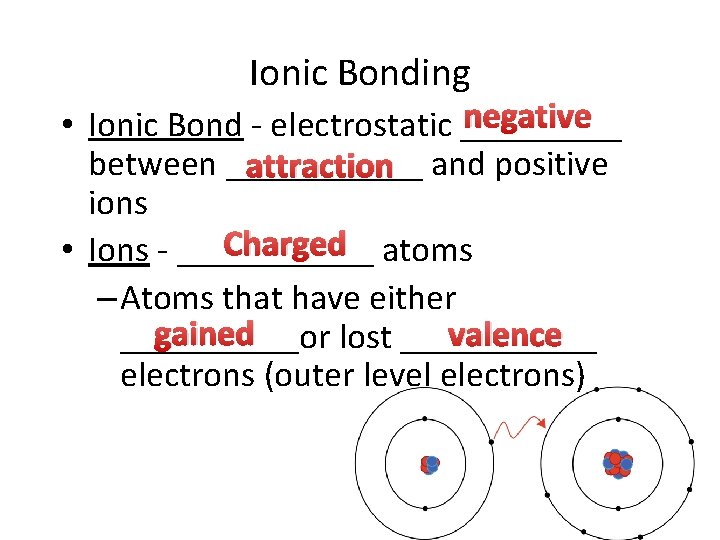
Ionic Bonding negative • Ionic Bond - electrostatic _____ between ______ attraction and positive ions Charged atoms • Ions - ______ – Atoms that have either gained valence _____or lost ______ electrons (outer level electrons)
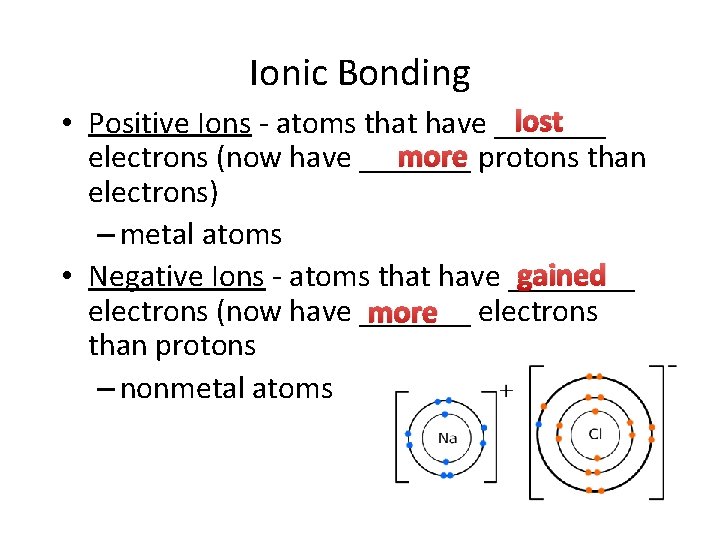
Ionic Bonding lost • Positive Ions - atoms that have _______ more protons than electrons (now have _______ electrons) – metal atoms gained • Negative Ions - atoms that have ____ electrons (now have _______ more electrons than protons – nonmetal atoms
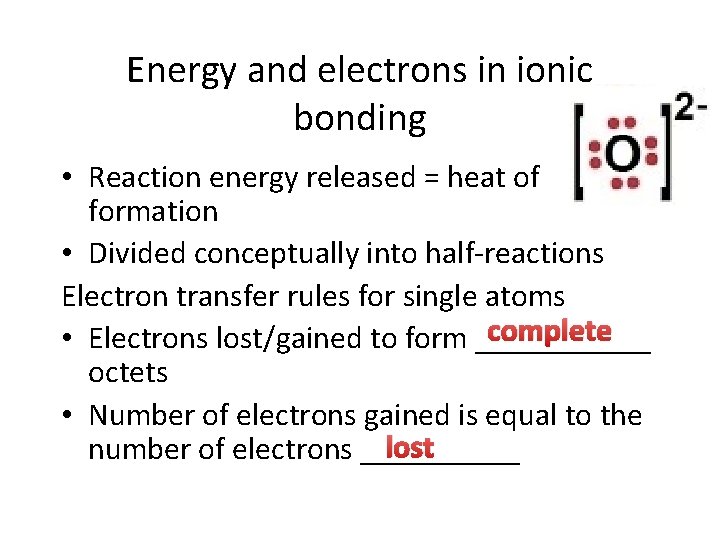
Energy and electrons in ionic bonding • Reaction energy released = heat of formation • Divided conceptually into half-reactions Electron transfer rules for single atoms complete • Electrons lost/gained to form ______ octets • Number of electrons gained is equal to the lost number of electrons _____
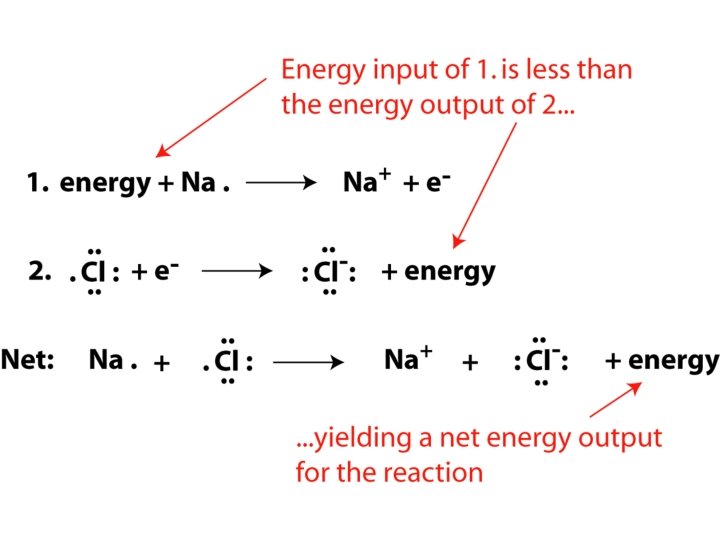
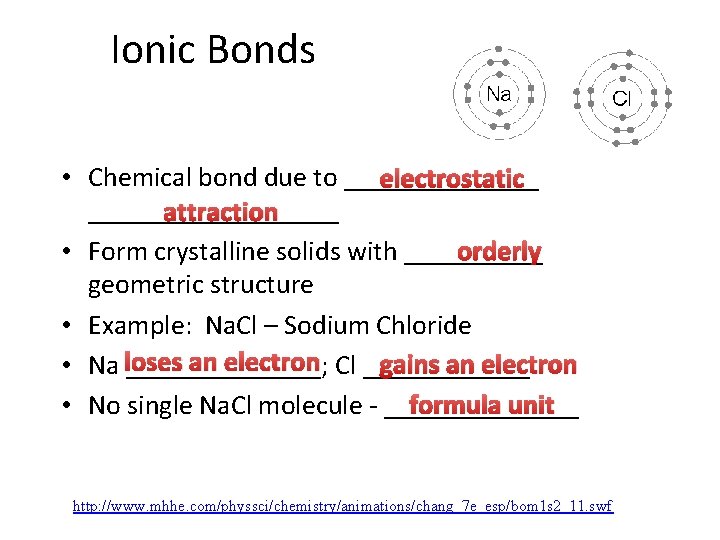
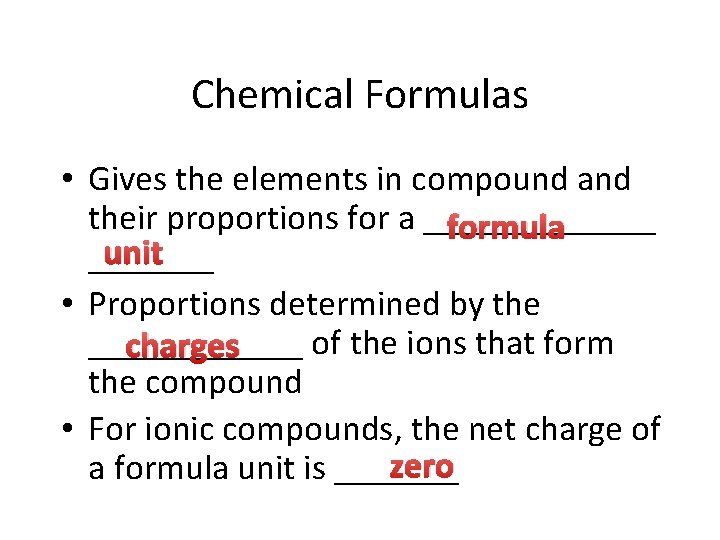
Ionic Bonds • Chemical bond due to _______ electrostatic _________ attraction • Form crystalline solids with _____ orderly geometric structure • Example: Na. Cl – Sodium Chloride an electron Cl ______ gains an electron • Na loses _______; formula unit • No single Na. Cl molecule - _______ http: //www. mhhe. com/physsci/chemistry/animations/chang_7 e_esp/bom 1 s 2_11. swf
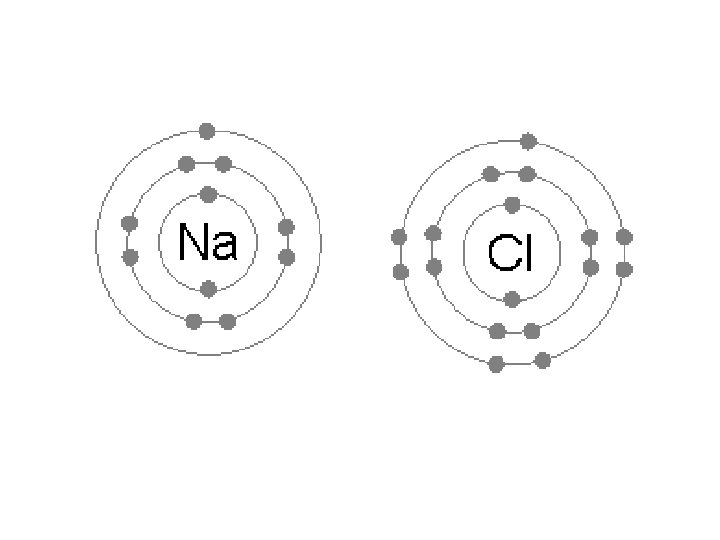
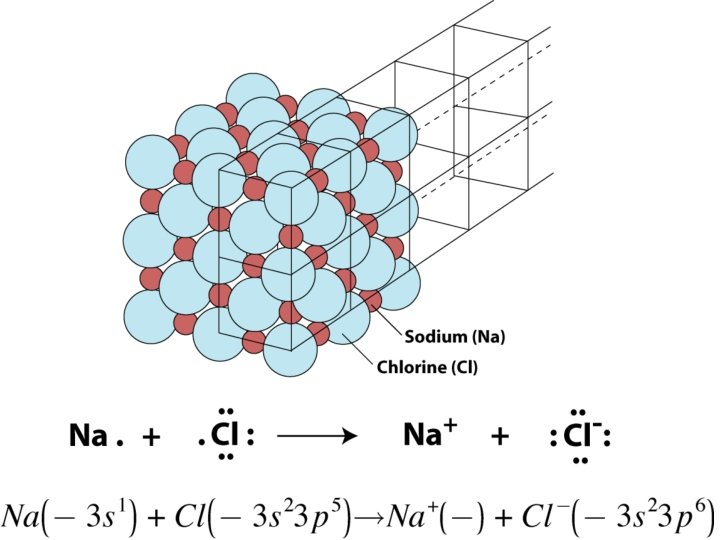

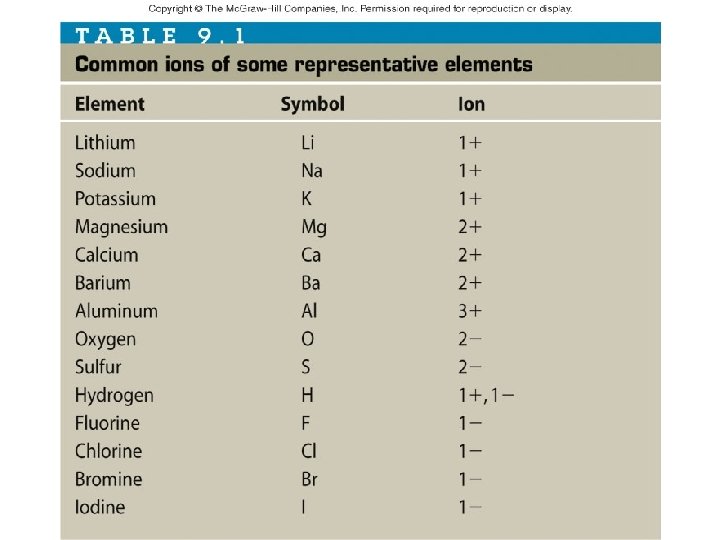
Ionic Compounds Ionic compounds ionic bonds • Characterized by ______ • White, crystalline solids soluble in water • Families IA and IIA lose electrons and form positive _____ ions • Families VIA and VIIA gain electrons to form negative ions _____
Chemical Formulas • Gives the elements in compound and their proportions for a _______ formula unit _______ • Proportions determined by the ______ of the ions that form charges the compound • For ionic compounds, the net charge of zero a formula unit is _______
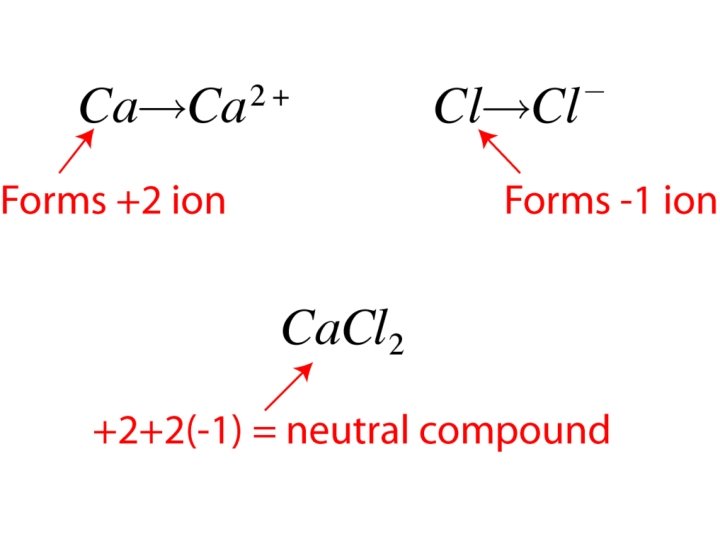
Ionic Compound Formulas • Two rules – Write symbol for _____ positive ion first followed by _____ negative ion symbol – Assign _____ subscripts to assure compound is electrically _____ neutral • Example: Calcium chloride

Writing Formulas for Ionic Compounds • Write the formulas for the following ionic compounds: KI – Potassium iodide ______ Mg. F 2 – Magnesium fluoride _____ Al. Br 3 – Aluminum bromide _____ Li 2 S – Lithium sulfide ______
Ionic compound names Nomenclature metal • Name of _______ (positive) ion first; then _____ (negative) ion, usually with an nonmetal -ide ending added to the name _______ • Ex. oxygen ____ oxide various • Some transition metals have ______ charges • Historical suffix usage – “-ic” for higher of two; “-ous” for lower • Modern approach – English name of metal followed by indicating charge
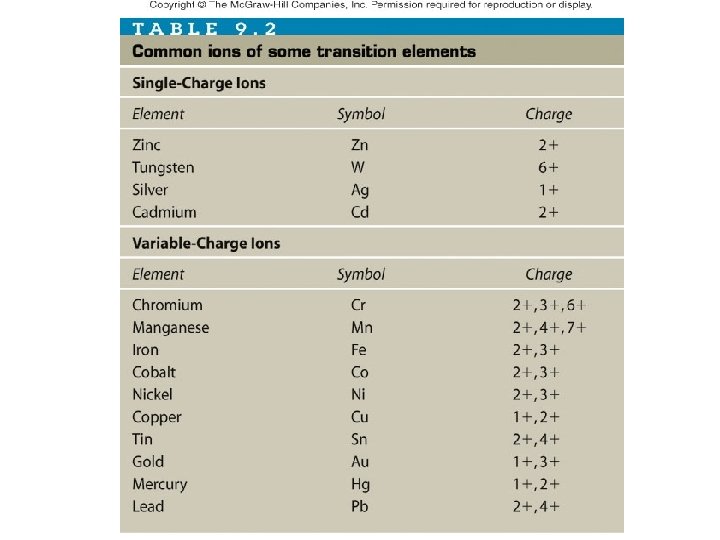
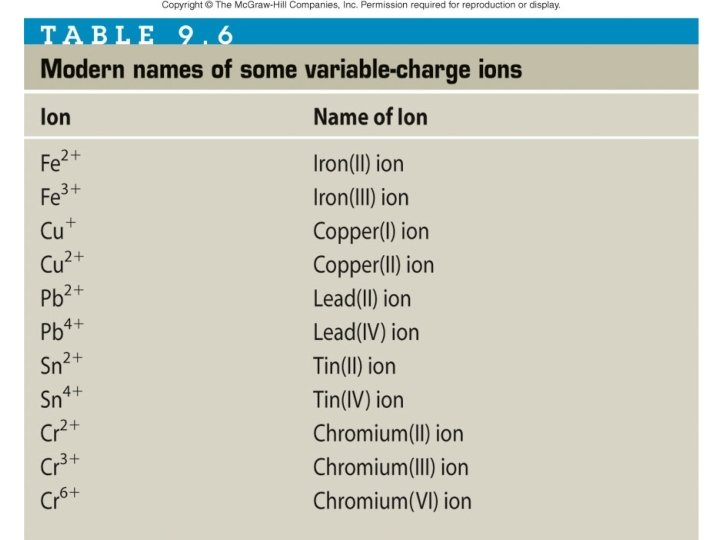
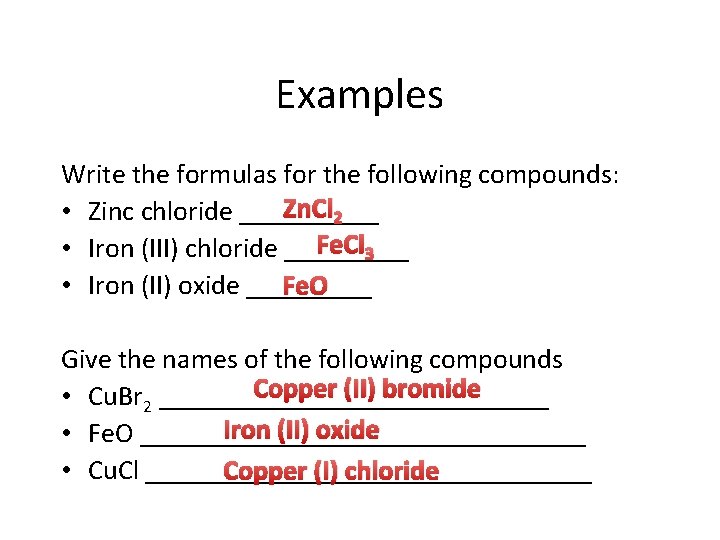
Examples Write the formulas for the following compounds: Zn. Cl 2 • Zinc chloride _____ Fe. Cl 3 • Iron (III) chloride _____ • Iron (II) oxide _____ Fe. O Give the names of the following compounds Copper (II) bromide • Cu. Br 2 ______________ Iron (II) oxide • Fe. O ________________ • Cu. Cl ________________ Copper (I) chloride
Polyatomic Ions • Some ions are formed by groups of atoms that are bonded together • These groups are referred to as polyatomic ions __________ – “poly-” means _______ many charge • These polyatomic ions carry a _____ and form ionic bonds just like single ions • The names and charges of the polyatomic ions do not need to be memorized and are used in naming the compounds and determining the formula of the compound. • When 2 or more polyatomic ions present, must be in parentheses.
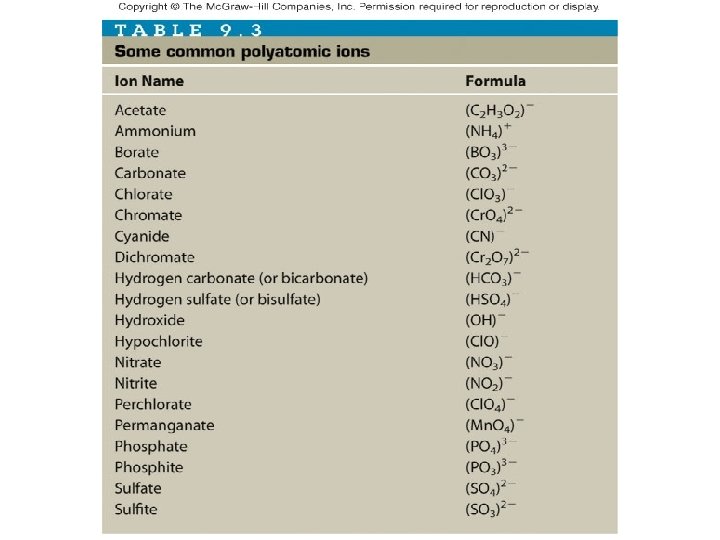
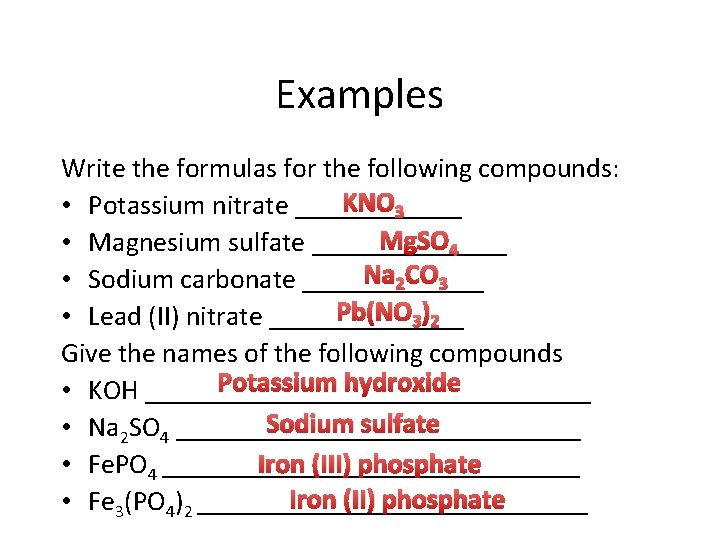
Examples Write the formulas for the following compounds: KNO 3 • Potassium nitrate ______ Mg. SO 4 • Magnesium sulfate _______ Na 2 CO 3 • Sodium carbonate _______ Pb(NO 3)2 • Lead (II) nitrate _______ Give the names of the following compounds Potassium hydroxide • KOH ________________ Sodium sulfate • Na 2 SO 4 _______________ Iron (III) phosphate • Fe. PO 4 _______________ Iron (II) phosphate • Fe 3(PO 4)2 ______________
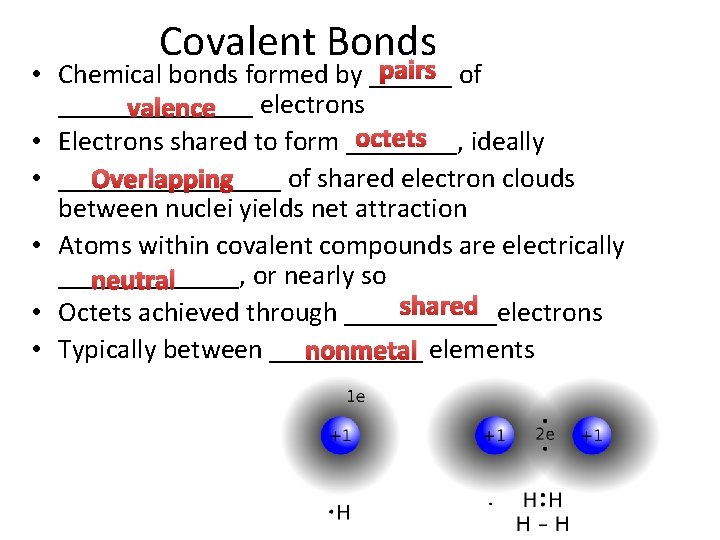
Covalent Bonds pairs of • Chemical bonds formed by __________ electrons valence octets • Electrons shared to form ____, ideally • ________ of shared electron clouds Overlapping between nuclei yields net attraction • Atoms within covalent compounds are electrically _______, or nearly so neutral shared • Octets achieved through ______electrons • Typically between ______ nonmetal elements
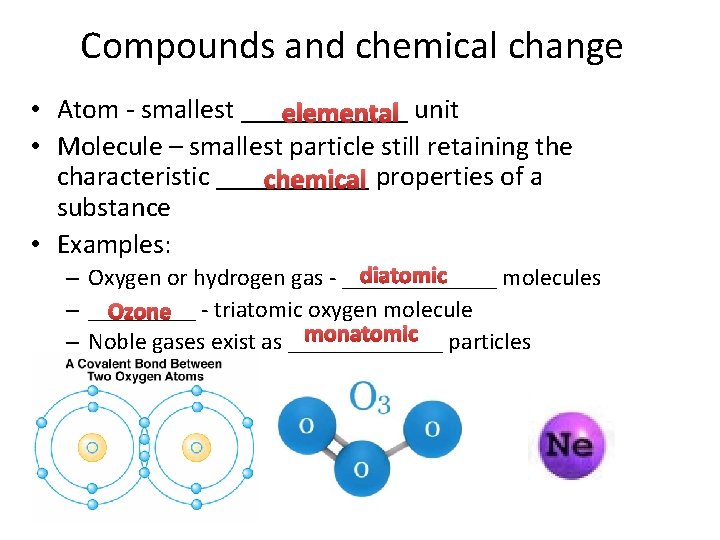
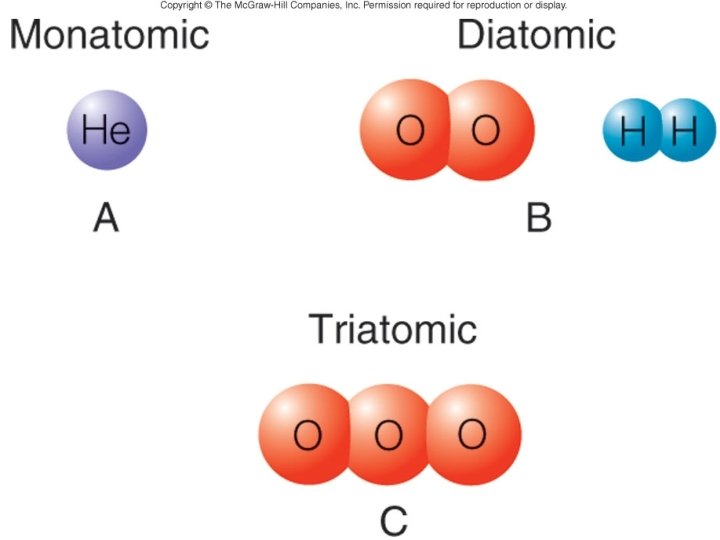
Compounds and chemical change • Atom - smallest ______ elemental unit • Molecule – smallest particle still retaining the characteristic ______ chemical properties of a substance • Examples: diatomic – Oxygen or hydrogen gas - _______ molecules – _____ Ozone - triatomic oxygen molecule monatomic particles – Noble gases exist as _______
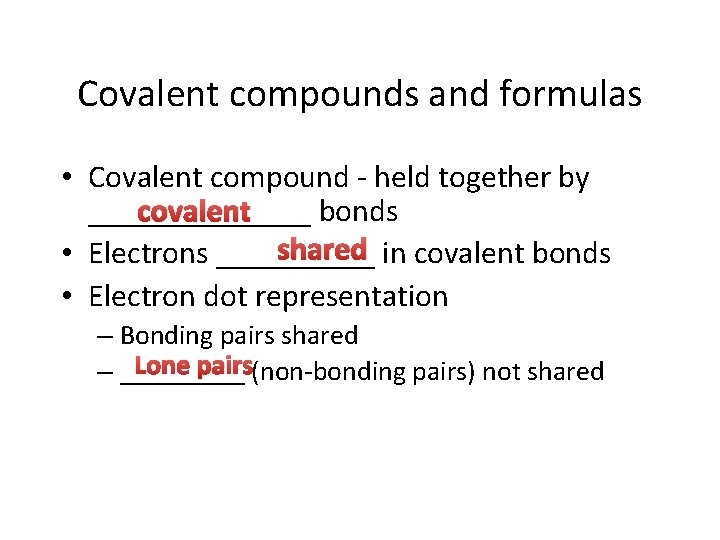
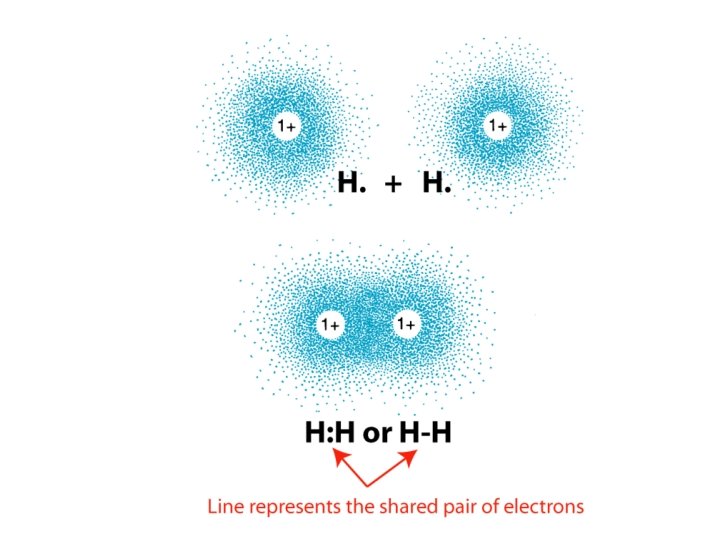
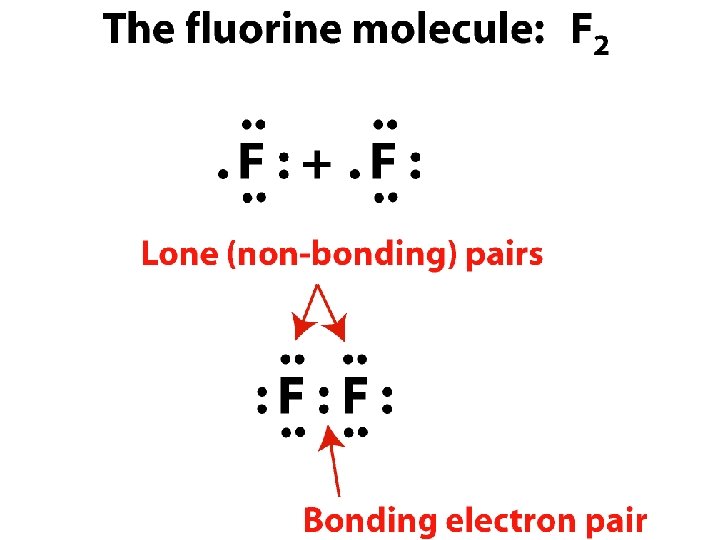
Covalent compounds and formulas • Covalent compound - held together by covalent _______ bonds shared in covalent bonds • Electrons _____ • Electron dot representation – Bonding pairs shared Lone pairs(non-bonding pairs) not shared – _____
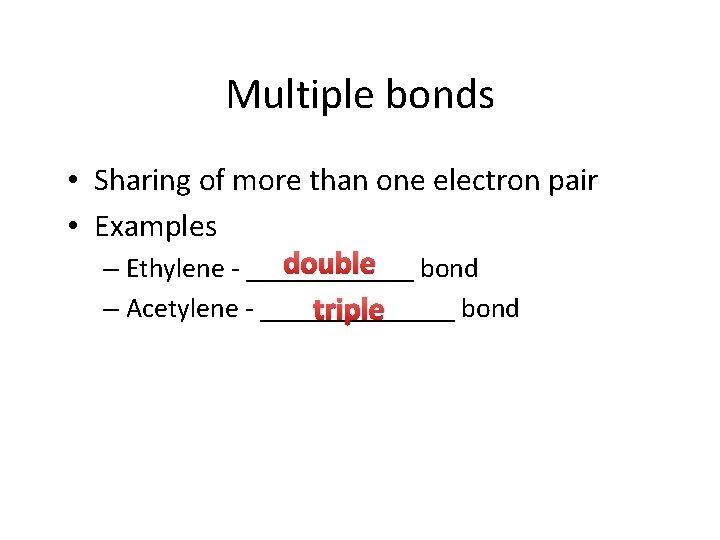
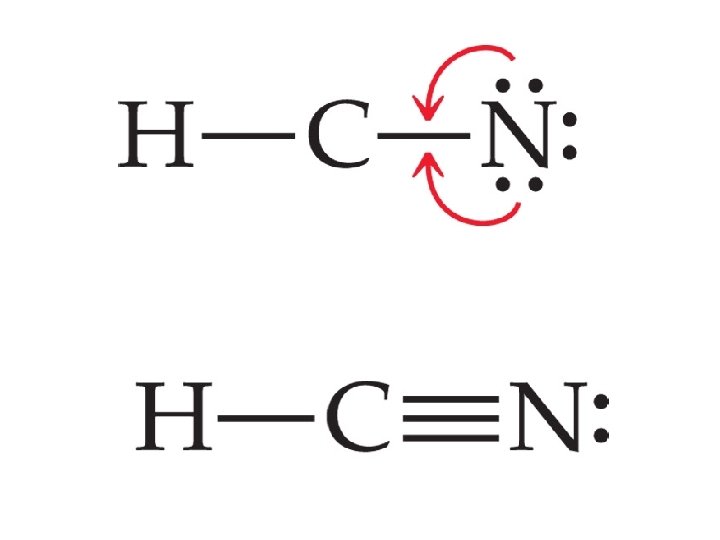
Multiple bonds • Sharing of more than one electron pair • Examples double bond – Ethylene - ______ – Acetylene - _______ bond triple

Multiple bonds
Covalent compound names Nomenclature 2 or more nonmetals • Composed of ___ • Same elements can combine to form a variety of different compounds – Examples: carbon dioxide or carbon monoxide • Two rules – First element in formula named with number of atoms indicated by ____ Greek prefix – Second element uses Greek prefix for number of atoms of that element and name of element ending in -ide _____ – Never use “mono” on first element if only 1
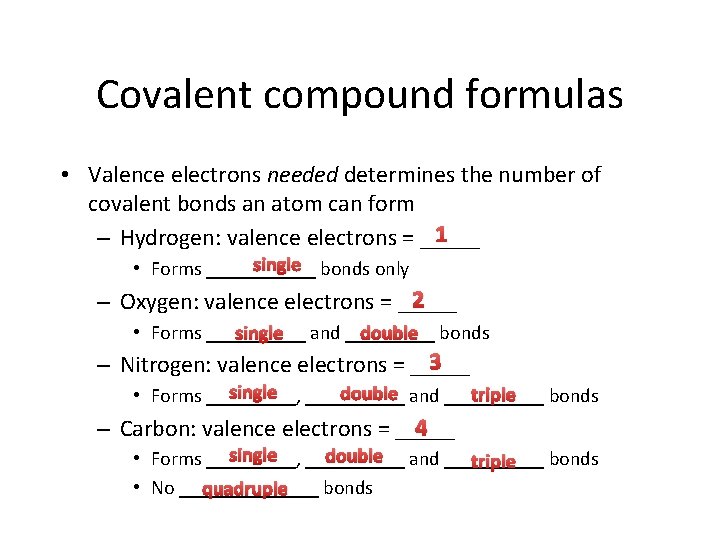
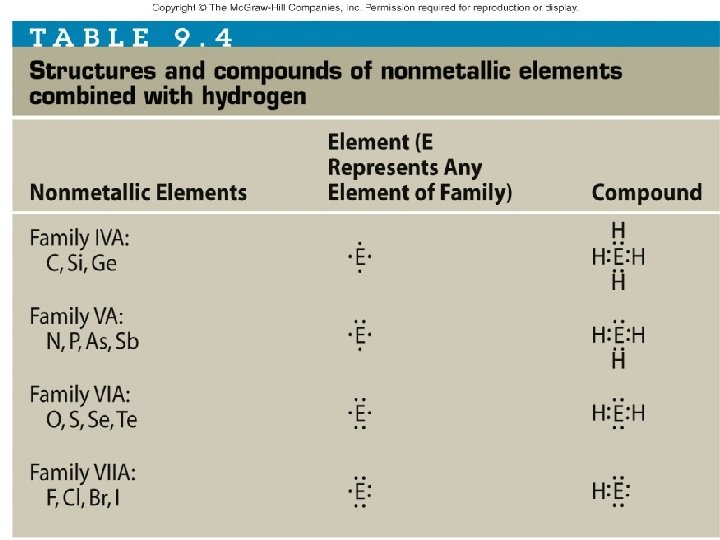
Covalent compound formulas • Valence electrons needed determines the number of covalent bonds an atom can form 1 – Hydrogen: valence electrons = _____ single bonds only • Forms ______ 2 – Oxygen: valence electrons = _____ • Forms _____ double bonds single and _____ 3 – Nitrogen: valence electrons = _____ single _____ double and _____ triple • Forms _____, bonds 4 – Carbon: valence electrons = _____ single _____ double and _____ • Forms _____, bonds triple • No _______ bonds quadruple
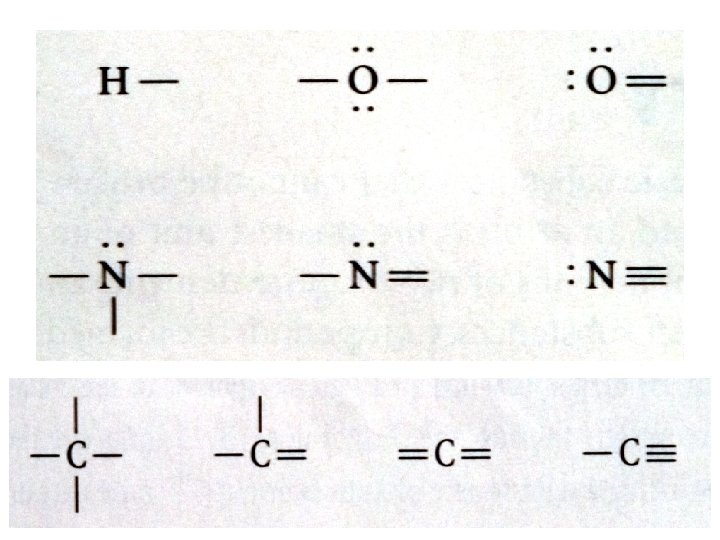
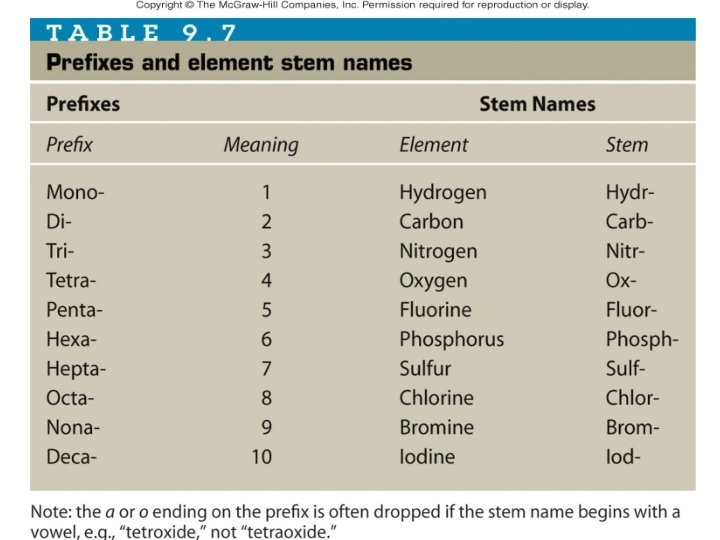
• Name the two compounds shown above Carbon dioxide • _______________ Carbon tetrachloride • _______________
• Give the names of the following compounds – NO 2 – _______________ Nitrogen dioxide – C 2 H 6 – ______________ Dicarbon hexahydride – N 2 O 4 – ______________ Dinitrogen tetroxide – SO 2 – _______________ Sulfur dioxide • Determine the formulas of the following compounds CBr 4 – Carbon tetrabromide _________ N 2 H 4 – Dinitrogen tetrahydride _________ – Tetraphosphorus pentoxide ________ P 4 O 5 C 3 H 8 – Tricarbon octahydride ________
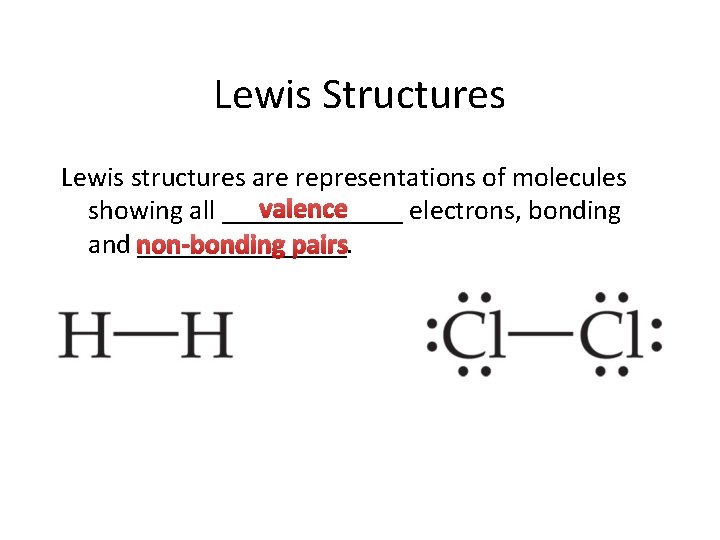
Lewis Structures Lewis structures are representations of molecules valence showing all _______ electrons, bonding and ________. non-bonding pairs

Writing Lewis Structures total of valence 1. Find the ____ electrons of all atoms in the molecule. – Use the ________ to Periodic table determine the number of valence electrons
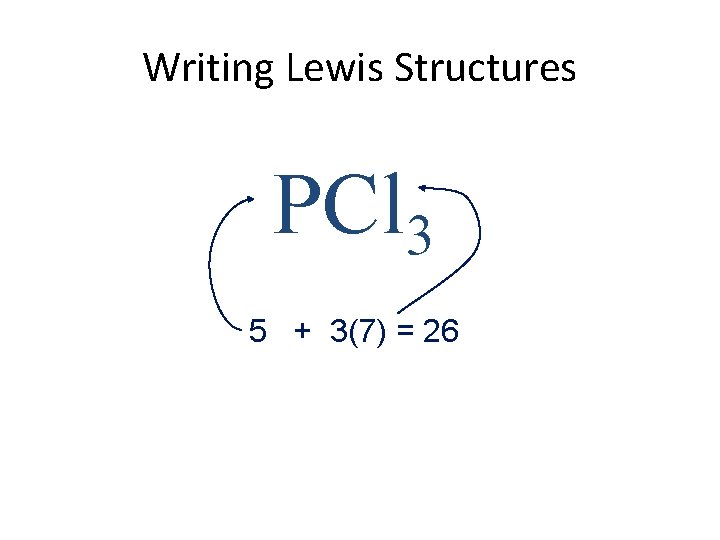
Writing Lewis Structures PCl 3 5 + 3(7) = 26
Writing Lewis Structures least 2. The central atom is the ______ electronegative element that isn’t hydrogen _______. single – Connect the outer atoms to it by _____ bonds, using a straight line. Each line represents the two electrons that are shared (6).
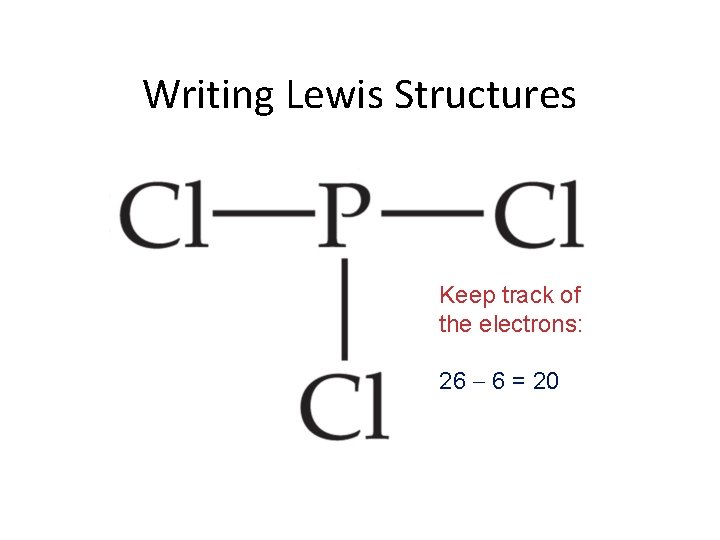
Writing Lewis Structures Keep track of the electrons: 26 6 = 20

Writing Lewis Structures outer atoms. (+18) 3. Fill the octets of the _______
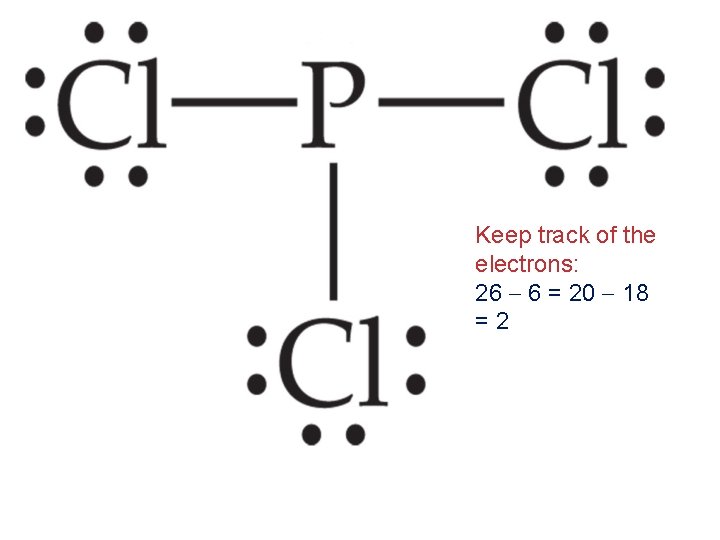
Keep track of the electrons: 26 6 = 20 18 =2

Writing Lewis Structures central atom. (+2) 4. Fill the octet of the _______
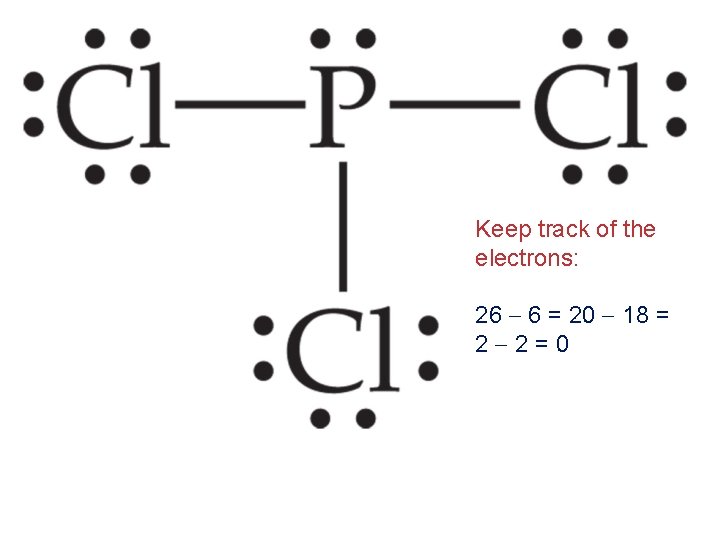
Keep track of the electrons: 26 6 = 20 18 = 2 2=0
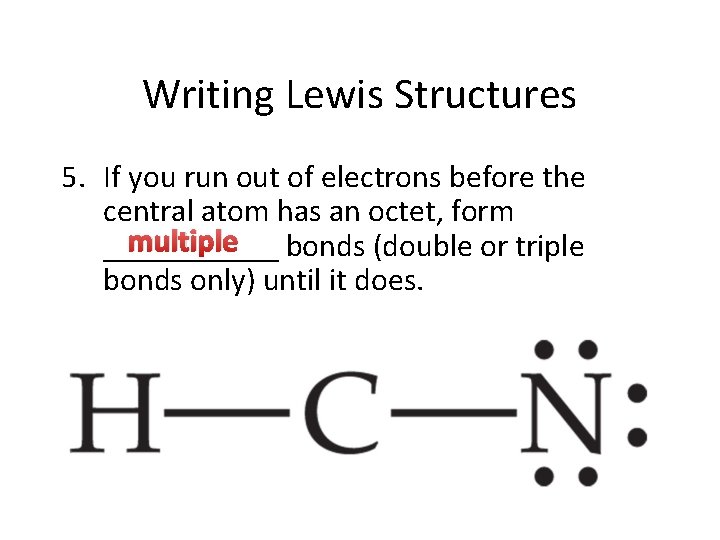
Writing Lewis Structures 5. If you run out of electrons before the central atom has an octet, form multiple bonds (double or triple ______ bonds only) until it does.
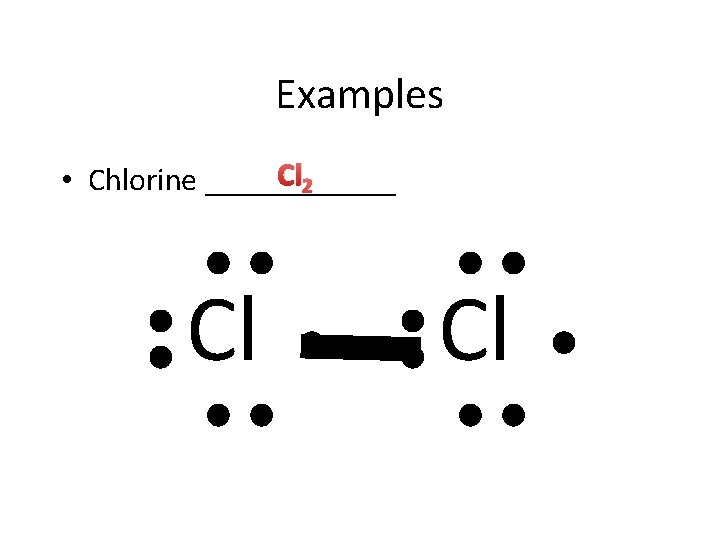
Examples Cl 2 • Chlorine ______ Cl Cl
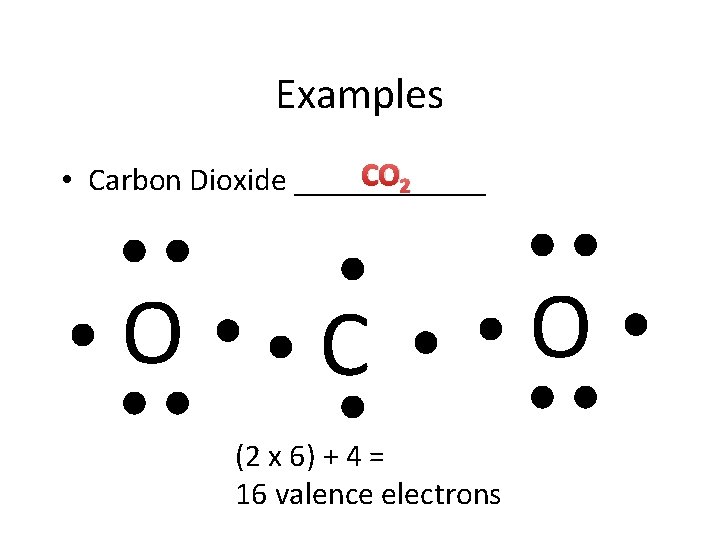
Examples CO 2 • Carbon Dioxide ______ O C (2 x 6) + 4 = 16 valence electrons O
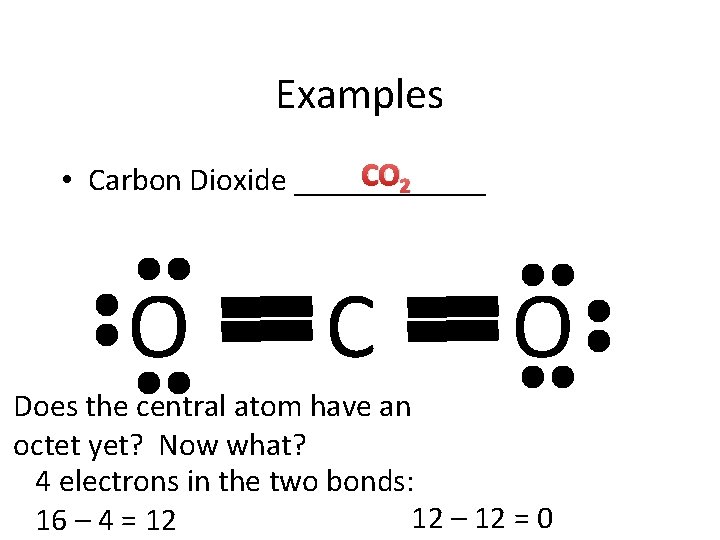
Examples CO 2 • Carbon Dioxide ______ O C O Does the central atom have an octet yet? Now what? 4 electrons in the two bonds: 12 – 12 = 0 16 – 4 = 12
Examples • Carbon Tetrachloride ______
Examples • Nitrogen Trihydride _______
Examples • C 2 H 4 – __________
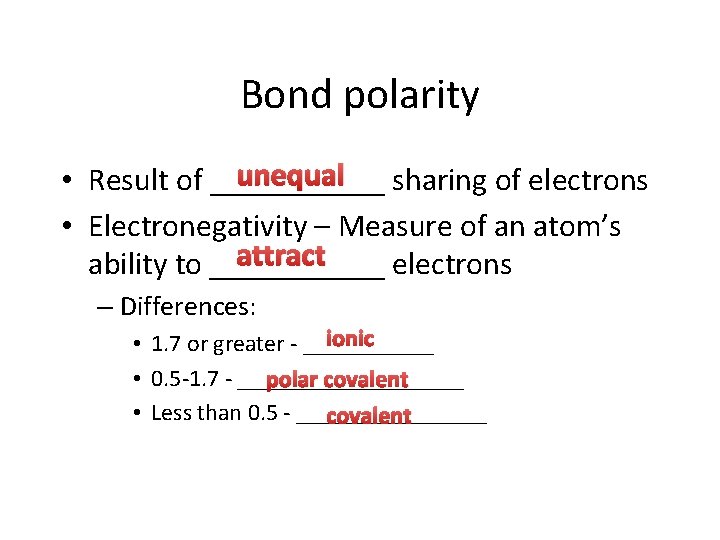
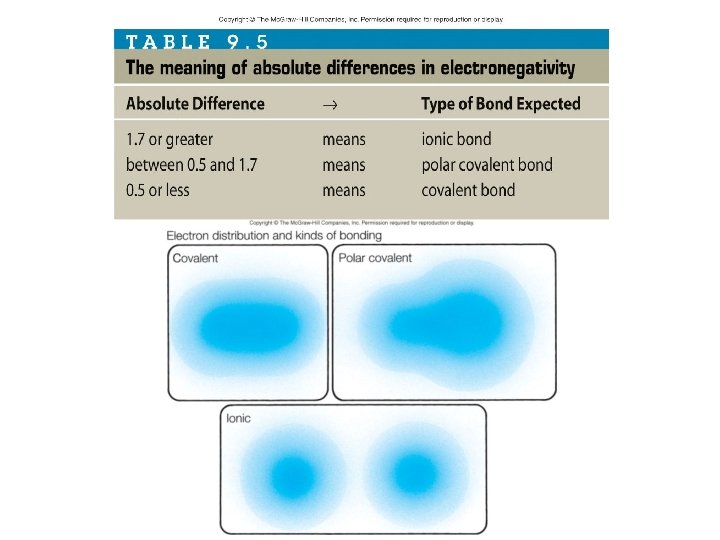
Bond polarity unequal sharing of electrons • Result of ______ • Electronegativity – Measure of an atom’s attract ability to ______ electrons – Differences: ionic • 1. 7 or greater - ______ • 0. 5 -1. 7 - __________ polar covalent • Less than 0. 5 - ________ covalent
- Slides: 58