Unit 1 Week 3 Thursday IONIC AND COVALENT












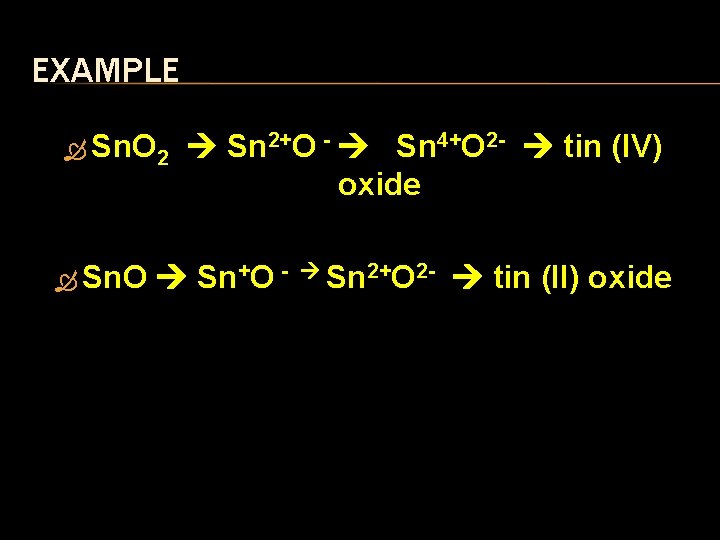




















- Slides: 60

Unit 1 Week 3 Thursday IONIC AND COVALENT BONDING AND NOMENCLATURE

IONIC NOMENCLATURE

NOMENCLATURE a branch of taxonomy concerned with the application of scientific names to taxa, based on a particular classification scheme and in accordance with agreed international rules and conventions

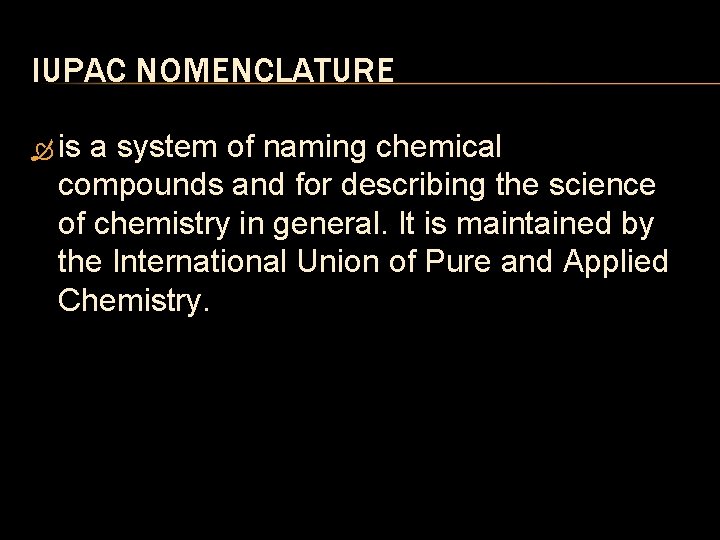
IUPAC NOMENCLATURE is a system of naming chemical compounds and for describing the science of chemistry in general. It is maintained by the International Union of Pure and Applied Chemistry.

IONIC BINARY COMPOUNDS Metal non-metal -ide

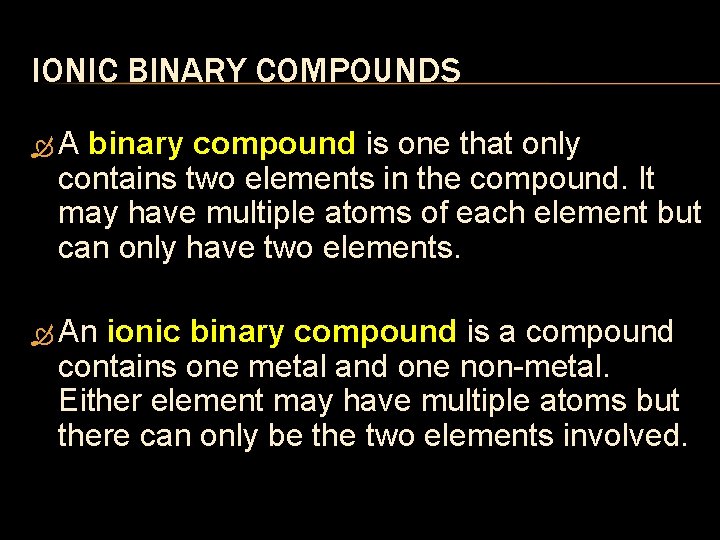
IONIC BINARY COMPOUNDS A binary compound is one that only contains two elements in the compound. It may have multiple atoms of each element but can only have two elements. An ionic binary compound is a compound contains one metal and one non metal. Either element may have multiple atoms but there can only be the two elements involved.

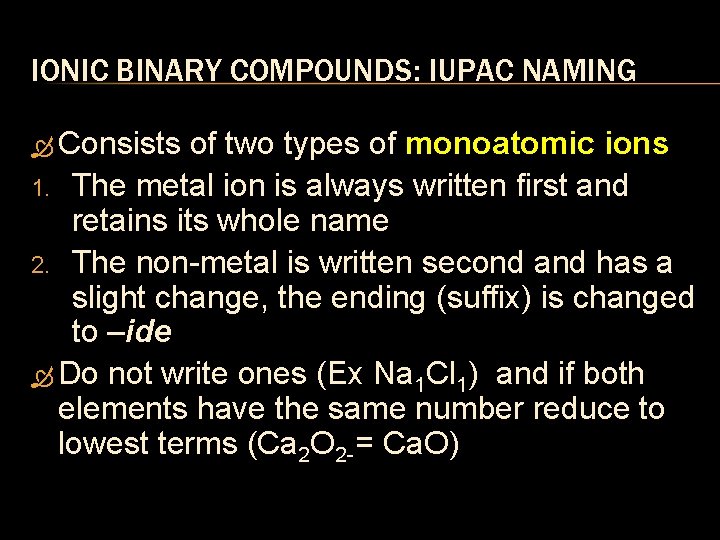
IONIC BINARY COMPOUNDS: IUPAC NAMING Consists of two types of monoatomic ions 1. The metal ion is always written first and retains its whole name 2. The non metal is written second and has a slight change, the ending (suffix) is changed to –ide Do not write ones (Ex Na 1 Cl 1) and if both elements have the same number reduce to lowest terms (Ca 2 O 2 = Ca. O)

EXAMPLE Example: Na+ Cl use the cross over method Na. Cl IUPAC name: sodium chloride The metal name is written in full and the non metal has the –ide suffix added to it. Sodium chloride

Binary compounds can be made up of more than two ions, provided that there are only two types of elements. Example: Al 2 O 3 STUDY TIP: All metals in group 1 and 2 follow periodic law. Check all the others metals when naming.

PRACTICE Work on the problems given on the sheet.

IONIC MULTIVALENT BINARY COMPOUNDS Metal (charge) non-metal-ide

IONIC MULTIVALENT BINARY COMPOUNDS A multivalent compound is one that may have varied numbers of electrons in its valence shell. This occurs with elements that fall outside of the representative elements. The transition metals are elements that commonly have multiple valence shell electrons. This means that they can form compounds in various proportions.

Example: Copper + Oxygen Copper and oxygen could have two different formulas with two completely different properties. Cu. O and Cu 2 O In order to differentiate the two compounds we must use a different method to name them to avoid confusion.

IONIC MULTIVALENT BINARY COMPOUNDS: IUPAC NAMING Same as Ionic Binary but it indicates the metals charge List the metal name first After the metal name indicate the ion charge in brackets using roman numerals. The non metal has ide suffix added.

Do not write 1’s and reduce when possible ONLY SHOW ROMAN NUMERALS FOR MULTIVALENT COMPOUNDS Not all transition metals are multivalent and thus do not have roman numerals
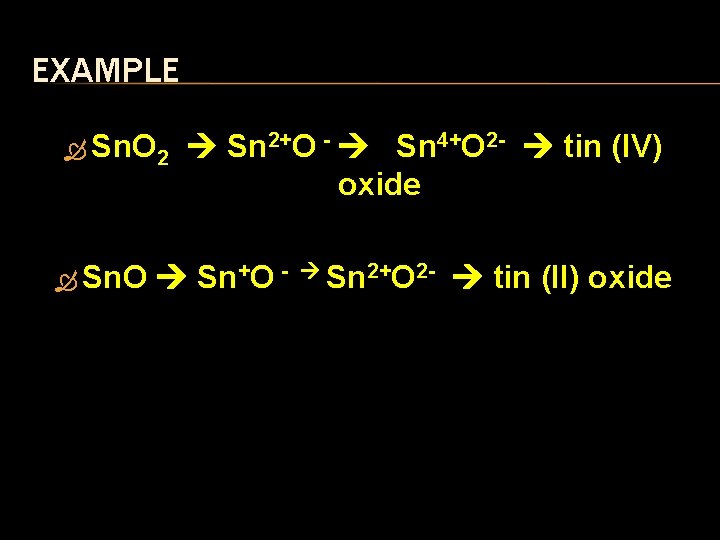
EXAMPLE Sn. O 2 Sn. O Sn 2+O - Sn 4+O 2 - tin (IV) oxide Sn+O - Sn 2+O 2 - tin (II) oxide

Work on the practice questions, They are homework

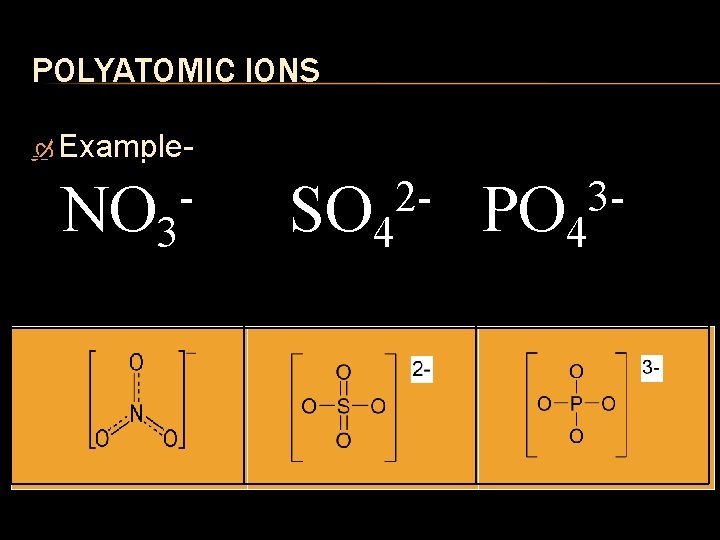
POLYATOMIC IONS

MONATOMIC IONS Ions that are composed of more than one atom are called monatomic ions. Monoatomic Ion an ion composed of only one atom. We have only looked at these so far

POLYATOMIC IONS Polyatomic Ions are ions that are composed of more than one atom. The entire molecule carries a charge to it.

POLYATOMIC IONS Example NO 3 2 SO 4 3 PO 4

BONDING Ionic Bonding with polyatomic ions occurs in the same manner as it does with binary atomic molecules. Use the crossover method Be sure that the charge that is crossed over applies to the whole polyatomic ion. If the charge is greater than 1, use brackets around the polyatomic ion to indicate the number applies to the whole ion.

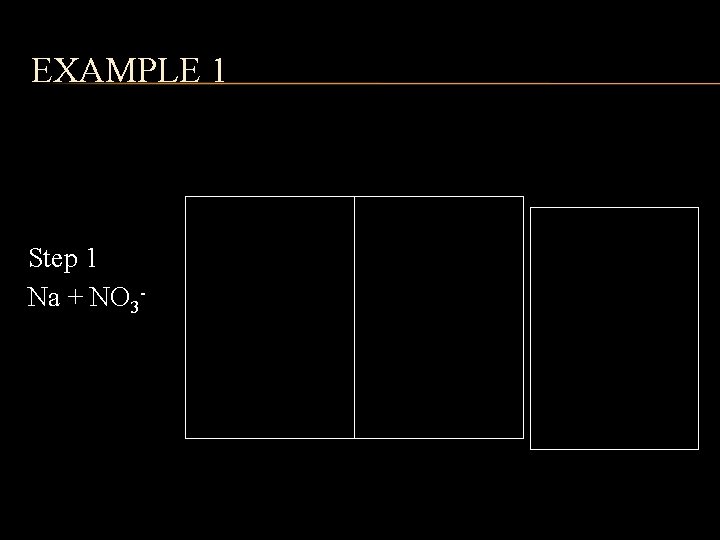
EXAMPLE 1 Step 1 Na + NO 3 - Step 2 1+ Na Step 3 1 - 1+ NO 3 Na 1 NO 3 Step 4 Na. NO 3

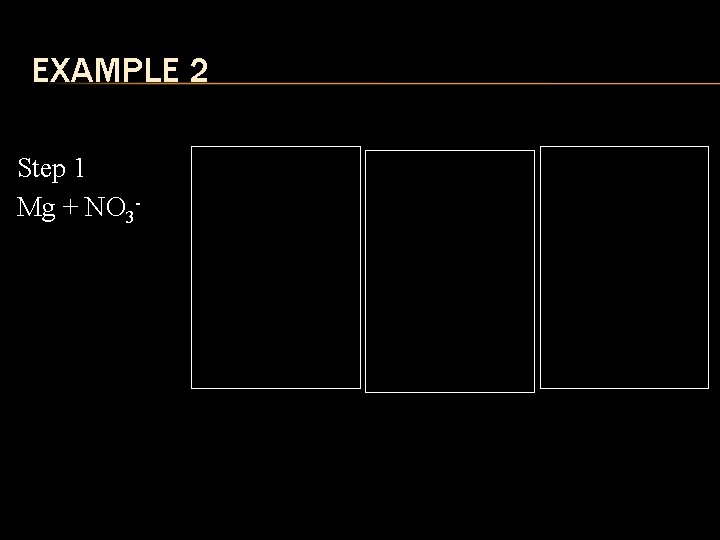
EXAMPLE 2 Step 1 Mg + NO 3 - Step 2 2+ Mg Step 3 12+ NO 3 Mg 1 NO 3 Step 4 Mg (NO 3)2

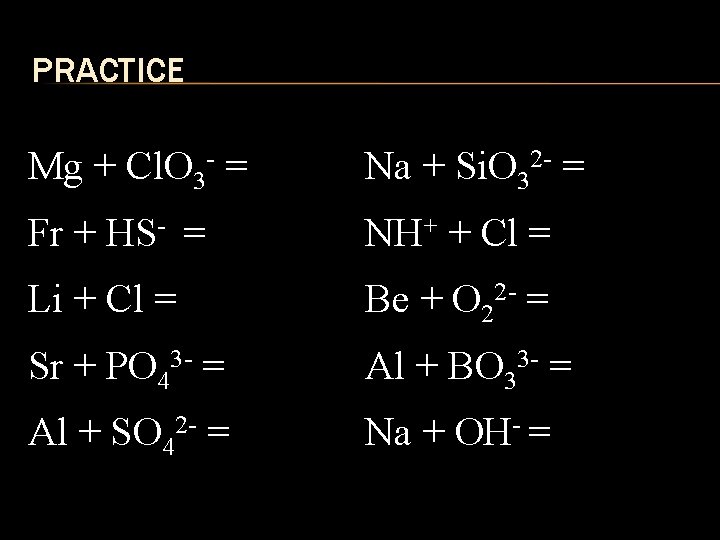
PRACTICE Mg + Cl. O 3 - = Na + Si. O 32 - = Fr + HS- = NH+ + Cl = Li + Cl = Be + O 22 - = Sr + PO 43 - = Al + BO 33 - = Al + SO 42 - = Na + OH- =

NAMING IONIC POLYATOMIC COMPOUNDS: IUPAC Multivalent: Metal (charge) polyatomic ion Monovalent: Metal polyatomic ion Tertiary ionic compounds are comprised of a metal ion and a polyatomic ion. Write the polyatomic ions in the same way as monatomic ions.

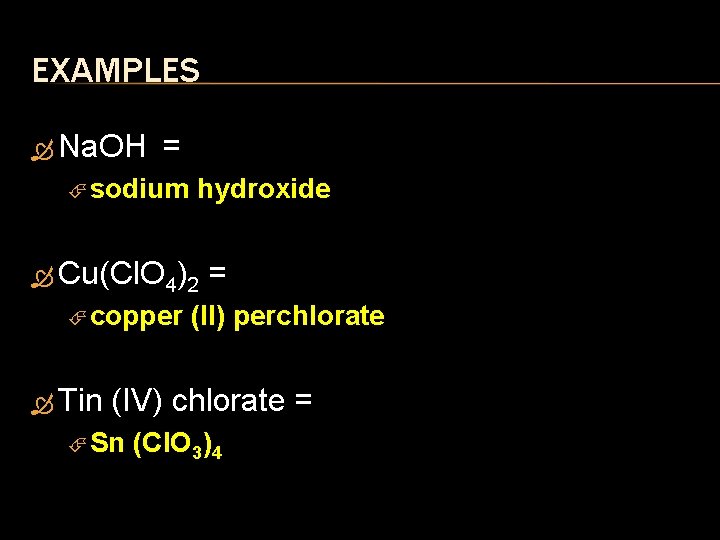
EXAMPLES Na. OH = sodium hydroxide Cu(Cl. O 4)2 = copper (II) perchlorate Tin (IV) chlorate = Sn (Cl. O 3)4

OXYANIONS Oxyanions = a polyatomic ion that includes oxygen. Their name depends on how many Oxygen’s it has in the poly atomic ion.

OXYANIONS Example Cl. O hypochlorite ion hypo _____ ite Cl. O 2 chlorite ion ______ ite Cl. O 3 chlorate ion _____ ate Cl. O 4 perchlorate ion per _______ ate This also applies to Br and I

PRACTICE Do the questions on the note.

COVALENT BONDS (P 36 -39)

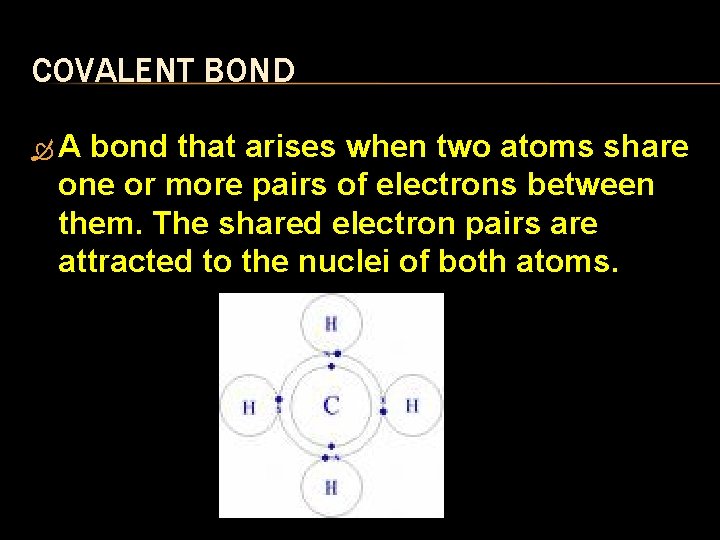
COVALENT BOND A bond that arises when two atoms share one or more pairs of electrons between them. The shared electron pairs are attracted to the nuclei of both atoms.

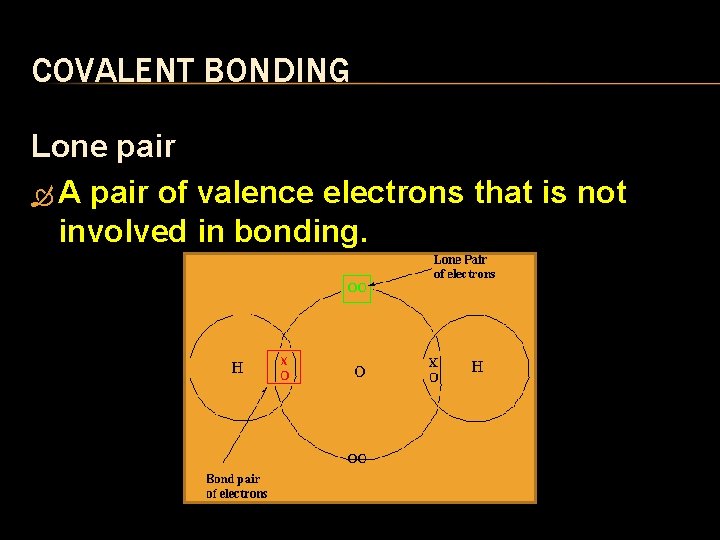
COVALENT BONDING Lone pair A pair of valence electrons that is not involved in bonding.

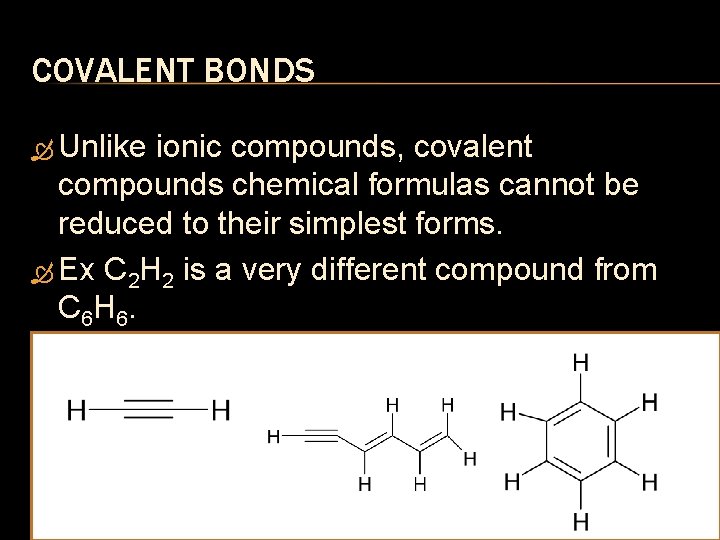
COVALENT BONDS Unlike ionic compounds, covalent compounds chemical formulas cannot be reduced to their simplest forms. Ex C 2 H 2 is a very different compound from C 6 H 6.

COVALENT BONDS Classification of covalent bond molecules Molecules are classified by the number of atoms they contain. Diatomic molecules only contain two molecules Ex. Carbon monoxide CO

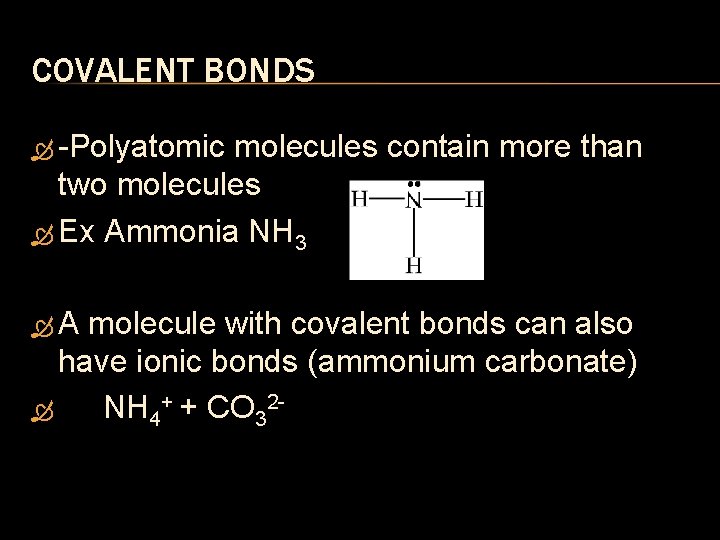
COVALENT BONDS Polyatomic molecules contain more than two molecules Ex Ammonia NH 3 A molecule with covalent bonds can also have ionic bonds (ammonium carbonate) NH 4+ + CO 32

ELEMENTS AS MOLECULES Some elements exist as molecules. diatomic H 2 and O 2 polyatomic S 8 and P 4

In ionic bonding atoms lost or gained electrons to form a stable octet for all of the molecules involved. Ex Mg 2+ Cl- = Mg. Cl 2 Na+ Cl- = Na. Cl For covalent bonds, atoms can share valence electrons to obtain a stable octet in all of the atoms.

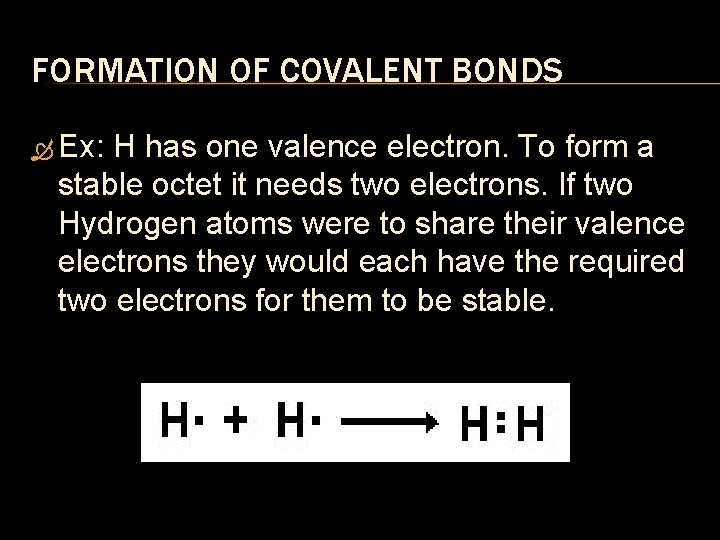
FORMATION OF COVALENT BONDS Ex: H has one valence electron. To form a stable octet it needs two electrons. If two Hydrogen atoms were to share their valence electrons they would each have the required two electrons for them to be stable.

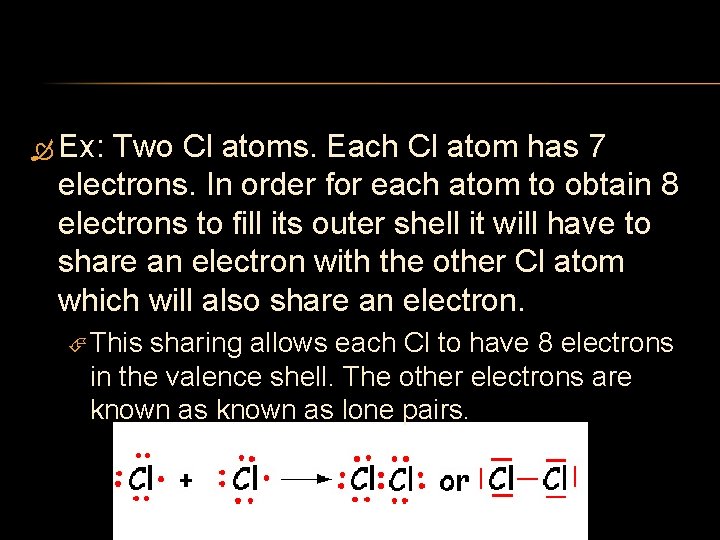
Ex: Two Cl atoms. Each Cl atom has 7 electrons. In order for each atom to obtain 8 electrons to fill its outer shell it will have to share an electron with the other Cl atom which will also share an electron. This sharing allows each Cl to have 8 electrons in the valence shell. The other electrons are known as lone pairs.

Octet Rule Elements will form bonds, so that in total they all have a full valence shell like a noble gas.

BONDING CAPACITY The number of electrons lost, gained or shared by an atom when it bonds chemically. Nitrogen has a bonding capacity of three (think back to its charge if it was ionic bonding) Examples: C=4 N=3 Halogen = 1 Hydrogen = 1 O=2

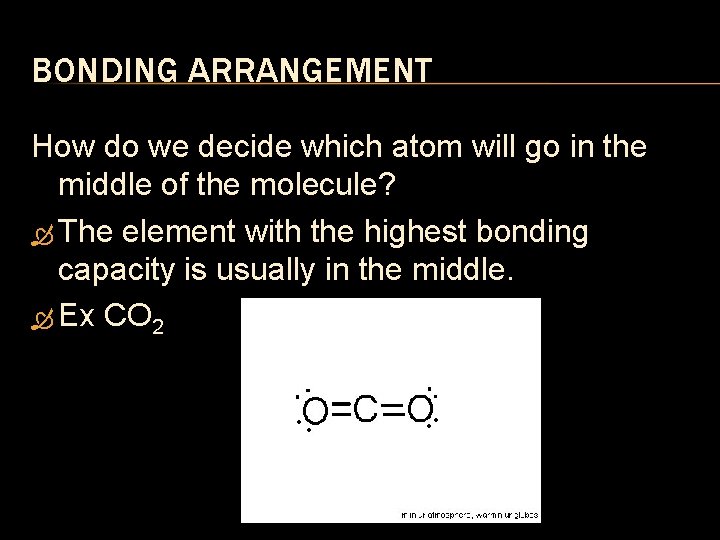
BONDING ARRANGEMENT How do we decide which atom will go in the middle of the molecule? The element with the highest bonding capacity is usually in the middle. Ex CO 2

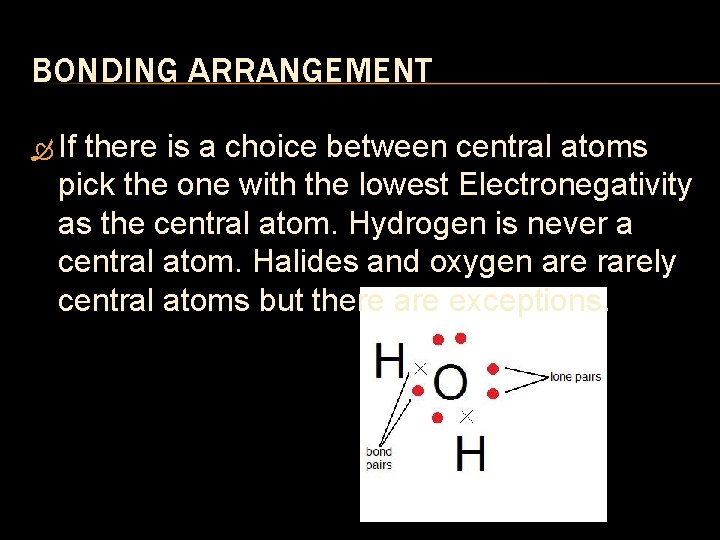
BONDING ARRANGEMENT If there is a choice between central atoms pick the one with the lowest Electronegativity as the central atom. Hydrogen is never a central atom. Halides and oxygen are rarely central atoms but there are exceptions.

QUESTIONS Page 39 # 1 4

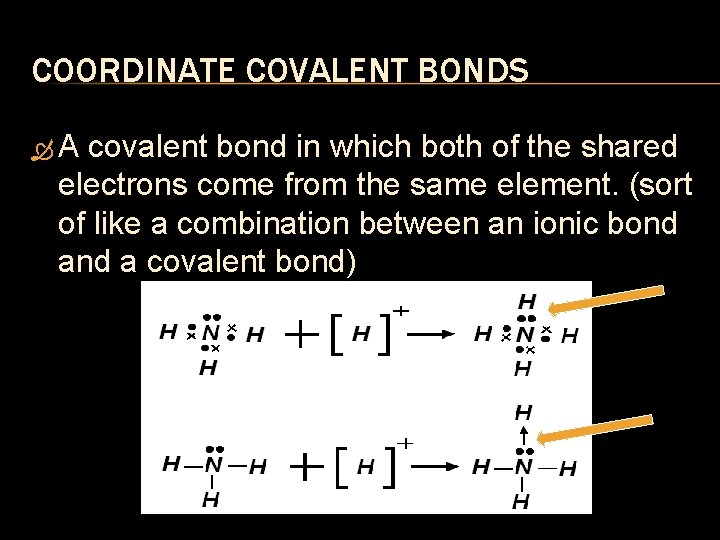
COORDINATE COVALENT BONDS A covalent bond in which both of the shared electrons come from the same element. (sort of like a combination between an ionic bond a covalent bond)

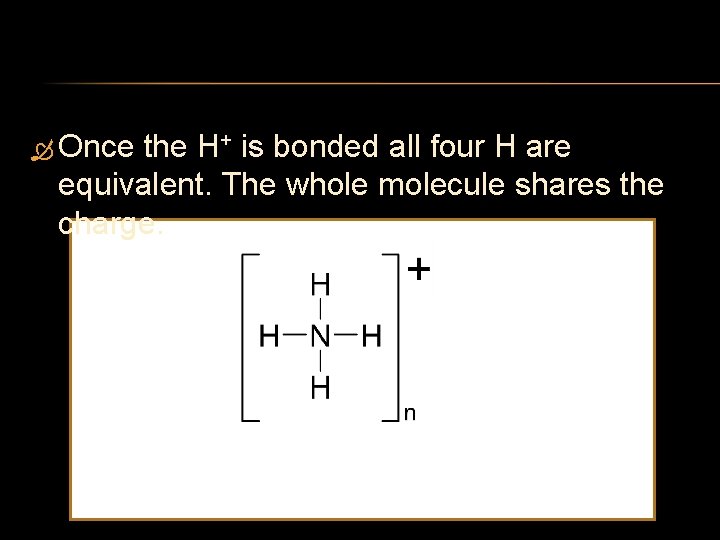
Once the H+ is bonded all four H are equivalent. The whole molecule shares the charge.

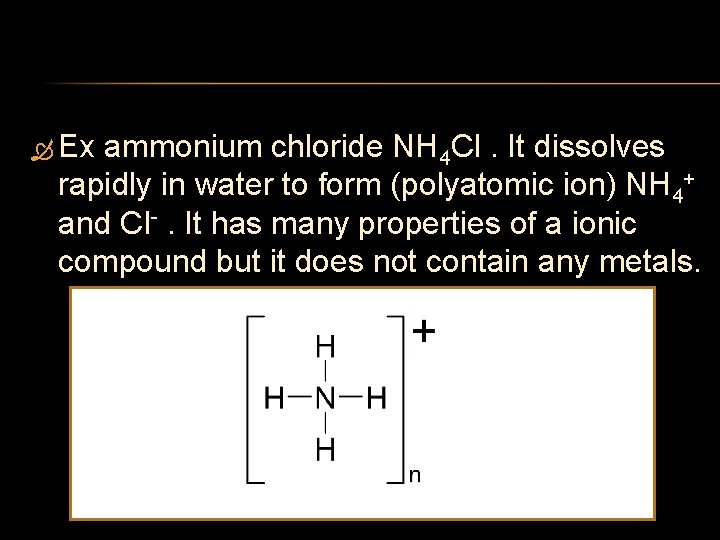
Ex ammonium chloride NH 4 Cl. It dissolves rapidly in water to form (polyatomic ion) NH 4+ and Cl . It has many properties of a ionic compound but it does not contain any metals.

STRENGTH They are strong; it requires a lot of energy to separate them. Therefore they are relatively stable at high temperatures. The strength of the bond increases as the number of electrons shared is added. Triple bonds > double bonds > single.

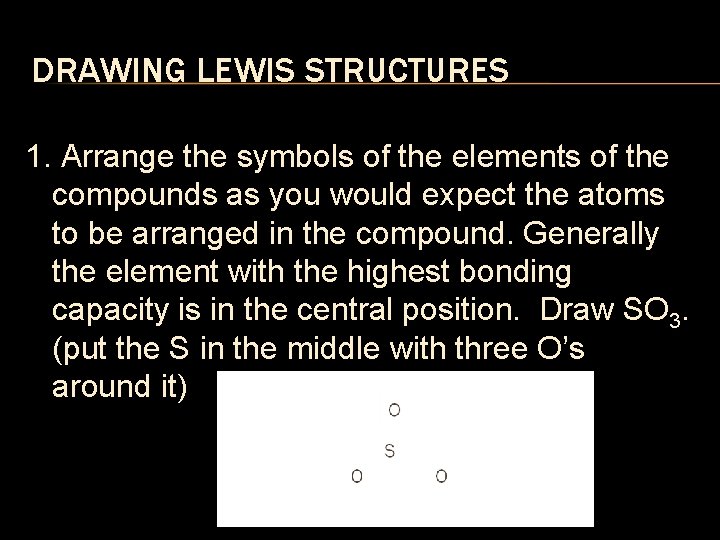
DRAWING LEWIS STRUCTURES 1. Arrange the symbols of the elements of the compounds as you would expect the atoms to be arranged in the compound. Generally the element with the highest bonding capacity is in the central position. Draw SO 3. (put the S in the middle with three O’s around it)

2. Add up the number of valence electrons in each atom. If it is a polyatomic ion, add one electron for each unit of negative charge, or subtract one for each unit of positive charge. Then add up the number of electrons needed for all of the elements to have a stable octet. Subtract the total number need by the total number and divide by two. This gives you the number of bonds needed.

What we need 4(8) = 32 What we have 3(6) O + 6 S = 24 Number of bonds (32 24) / 2 = 8/2 = 4 bonds It is divided by two because there are two electrons needed for each bond.

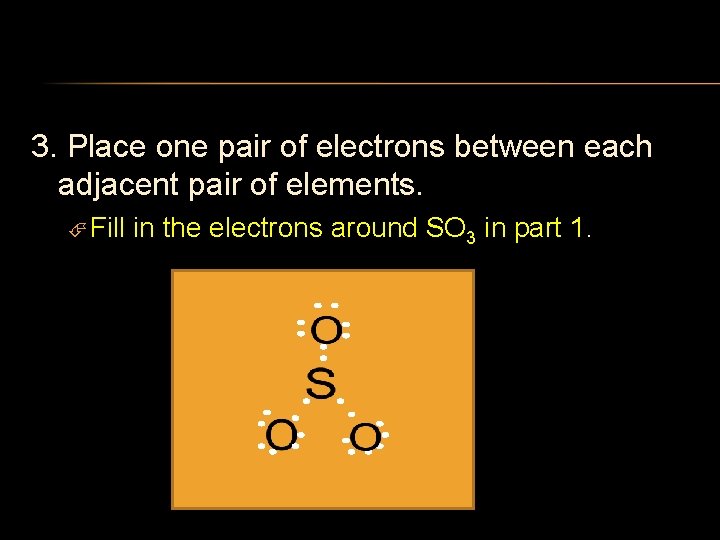
3. Place one pair of electrons between each adjacent pair of elements. Fill in the electrons around SO 3 in part 1.

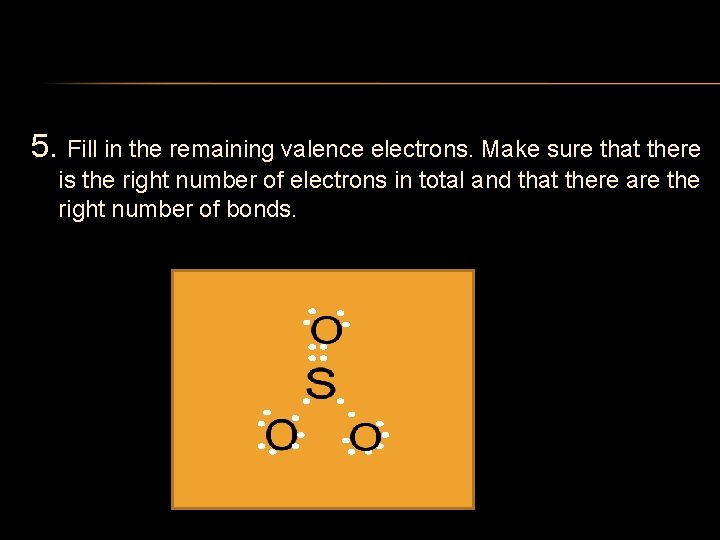
5. Fill in the remaining valence electrons. Make sure that there is the right number of electrons in total and that there are the right number of bonds.

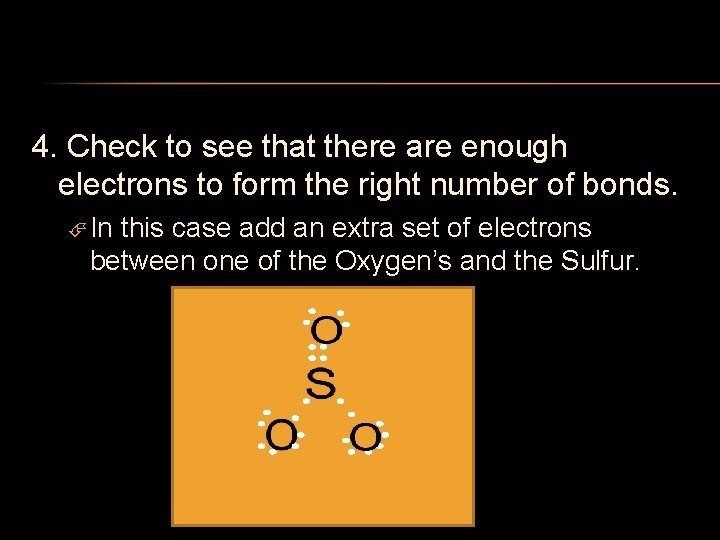
4. Check to see that there are enough electrons to form the right number of bonds. In this case add an extra set of electrons between one of the Oxygen’s and the Sulfur.

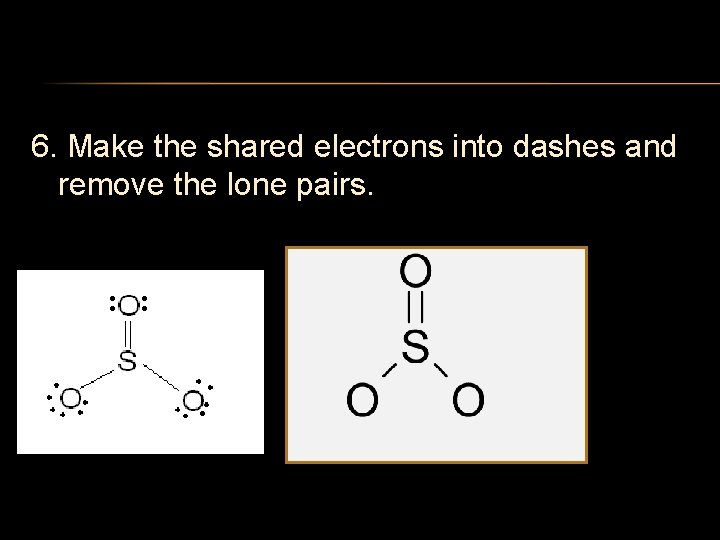
6. Make the shared electrons into dashes and remove the lone pairs.

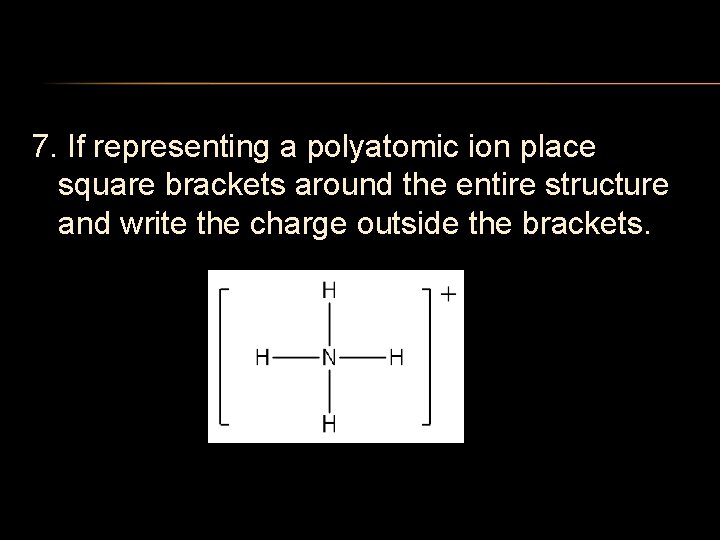
7. If representing a polyatomic ion place square brackets around the entire structure and write the charge outside the brackets.

STRUCTURAL FORMULA The lone pairs are not indicated and thus only the bonds sharing electrons are shown. Atoms that only need one valence electron will generally form single bonds where atoms that need two electrons will generally form two bonds. O 2 as an example. Draw the full Lewis structure. N 2 with its triple bonds.

More examples on the board

HOME WORK Ionic Nomenclature practice Ionic polyatomic nomenclature Lewis structures Covalent nomenclature –opps, next class Take home quiz – hand in REMINDER – Hand in Line spectra Lab