Unit 1 Topic 3 Kinetic Energy Words What




- Slides: 9

Unit 1 Topic 3 Kinetic Energy

Words? • What does kinetic mean to you? ki·net·ic/kəˈnetik/ Adjective: Of, relating to, or resulting from motion. (of a work of art) Depending on movement for its effect. Watch this! Video What is this called? Xbox KINECT

• What does energy mean to you? • What are some synonyms for ENERGY? en·er·gy/ˈenərjē Noun: The strength and vitality required for sustained physical or mental activity. A feeling of possessing such strength and vitality. Synonyms: power - vigour - vigor - vim - zip - strength - pep

• The Kinetic Theory states that all matter is in constant motion. • This means that all atoms and molecules are in constant motion in all states of matter. FASTER • Gas particles are moving ______ than liquid particles and solid molecules are moving ______. SLOWEST

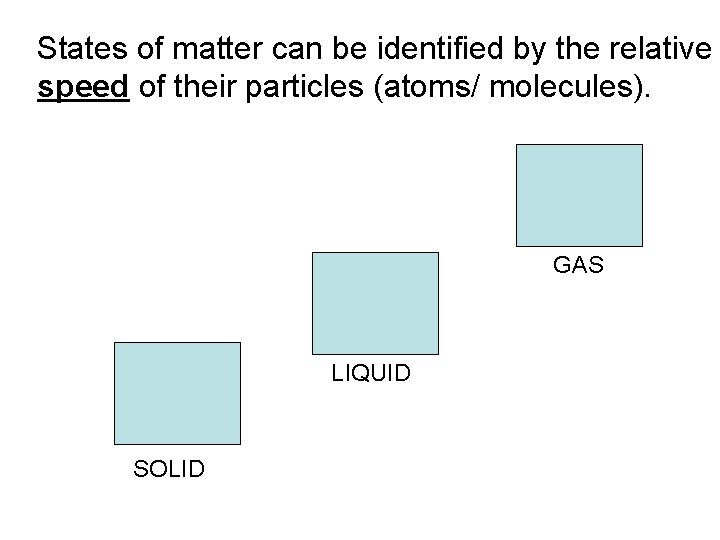
States of matter can be identified by the relative speed of their particles (atoms/ molecules). GAS LIQUID SOLID

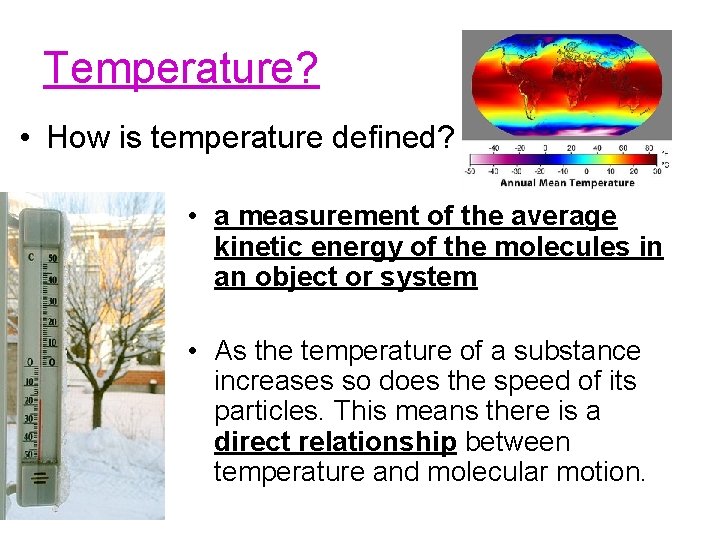
Temperature? • How is temperature defined? • a measurement of the average kinetic energy of the molecules in an object or system • As the temperature of a substance increases so does the speed of its particles. This means there is a direct relationship between temperature and molecular motion.

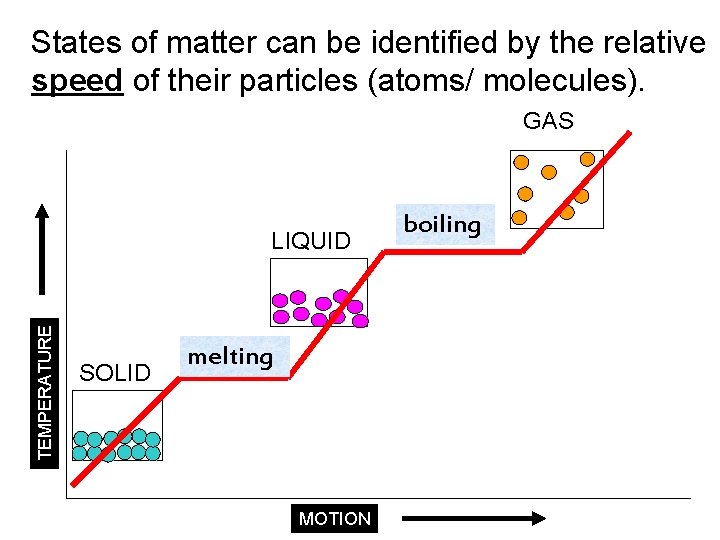
States of matter can be identified by the relative speed of their particles (atoms/ molecules). GAS TEMPERATURE LIQUID SOLID melting MOTION boiling

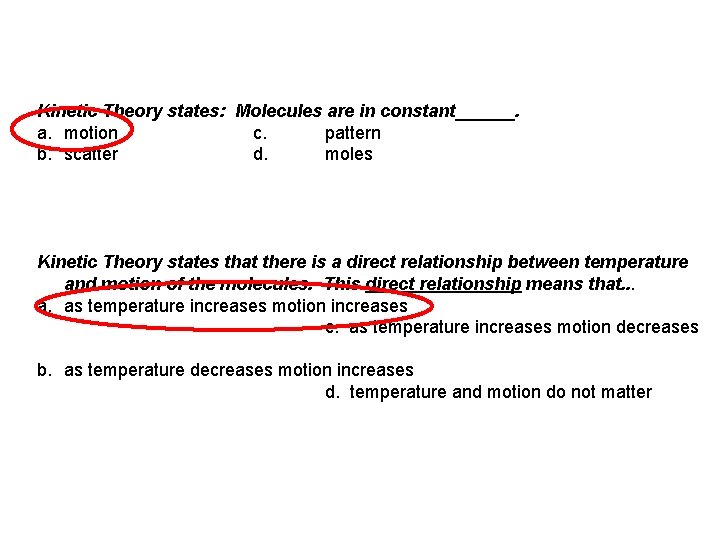
Kinetic Theory states: Molecules are in constant______. a. motion c. pattern b. scatter d. moles Kinetic Theory states that there is a direct relationship between temperature and motion of the molecules. This direct relationship means that. . . a. as temperature increases motion increases c. as temperature increases motion decreases b. as temperature decreases motion increases d. temperature and motion do not matter

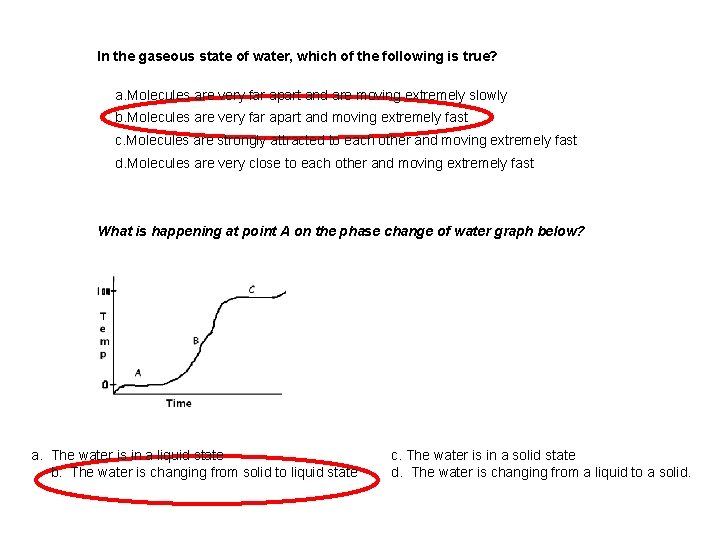
In the gaseous state of water, which of the following is true? a. Molecules are very far apart and are moving extremely slowly b. Molecules are very far apart and moving extremely fast c. Molecules are strongly attracted to each other and moving extremely fast d. Molecules are very close to each other and moving extremely fast What is happening at point A on the phase change of water graph below? a. The water is in a liquid state b. The water is changing from solid to liquid state c. The water is in a solid state d. The water is changing from a liquid to a solid.