Unit 1 The Building Blocks of Chemistry Section
Unit 1: The Building Blocks of Chemistry Section 1: Atoms
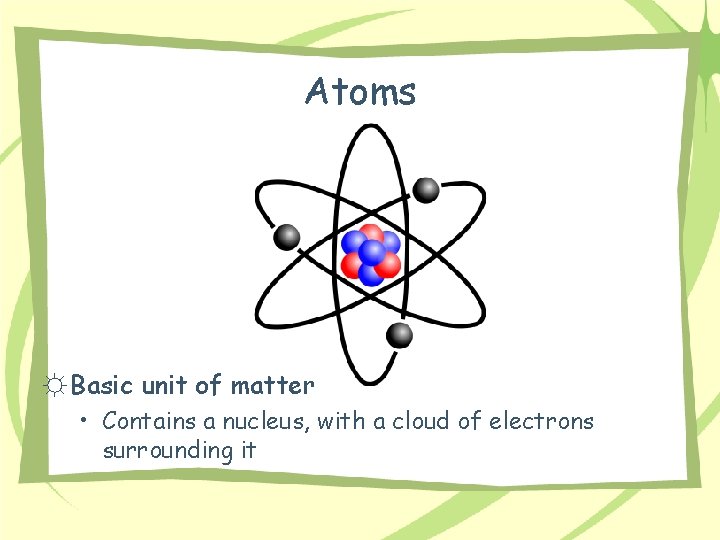
Atoms ☼ Basic unit of matter • Contains a nucleus, with a cloud of electrons surrounding it
Cornell Notes Topic: Details: Atoms ☼Basic unit of matter Symbols, questions, diagrams? • Contains a nucleus, with a cloud of electrons surrounding it Summary: Summarize the main ideas
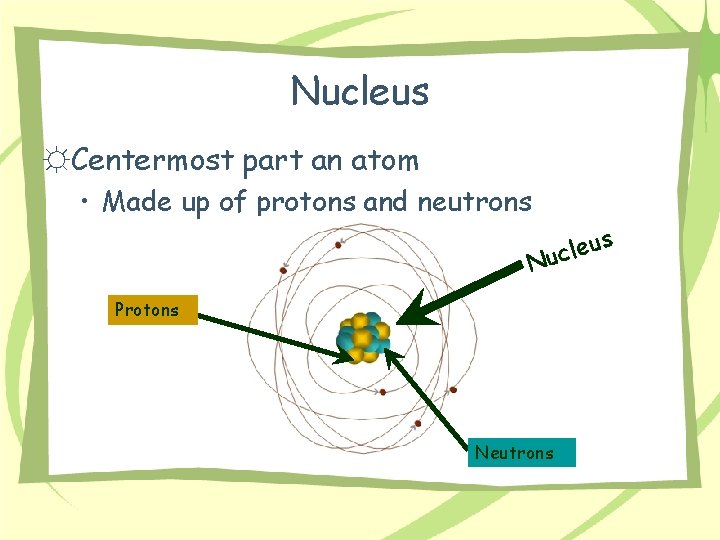
Nucleus ☼Centermost part an atom • Made up of protons and neutrons N s u e l uc Protons Neutrons
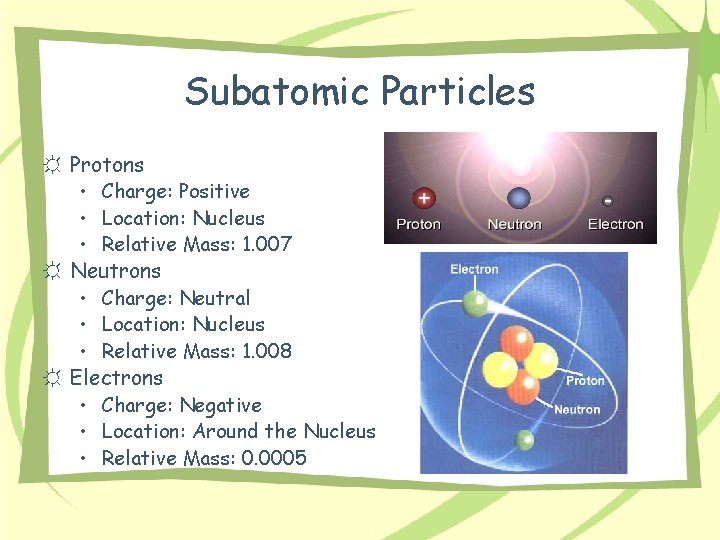
Subatomic Particles ☼ Protons • Charge: Positive • Location: Nucleus • Relative Mass: 1. 007 ☼ Neutrons • Charge: Neutral • Location: Nucleus • Relative Mass: 1. 008 ☼ Electrons • Charge: Negative • Location: Around the Nucleus • Relative Mass: 0. 0005

Mass vs. Size ☼Nucleus is the heaviest, but smallest region of the atom ☼Electrons are the lightest, but largest region of the atom
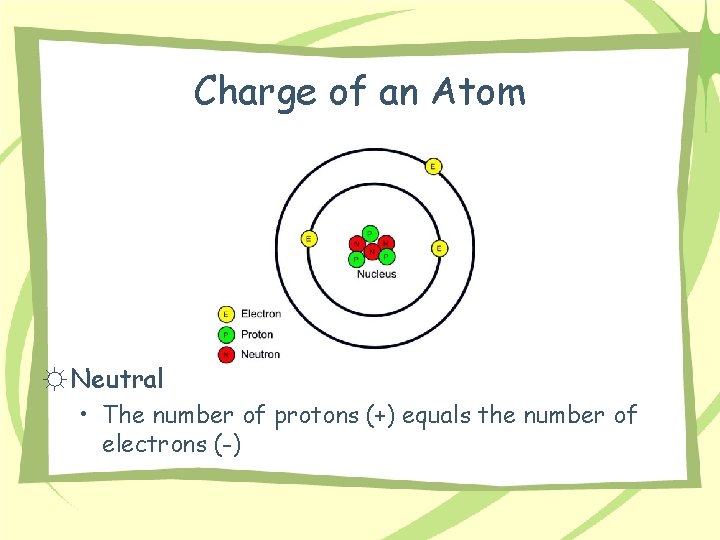
Charge of an Atom ☼ Neutral • The number of protons (+) equals the number of electrons (-)
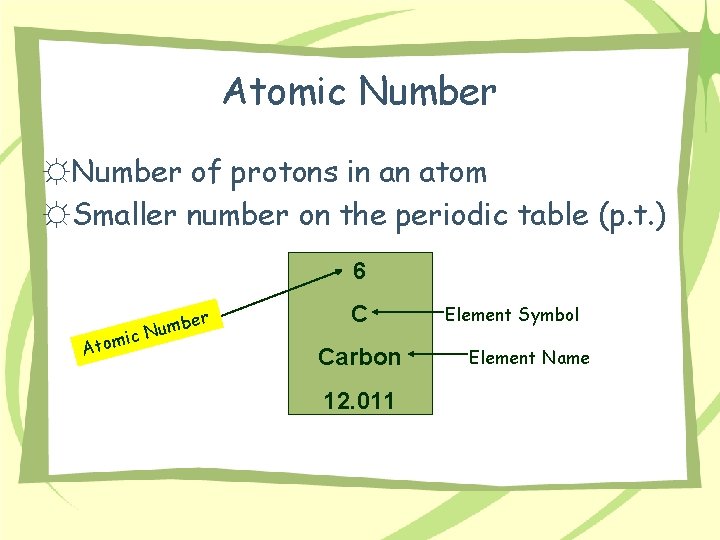
Atomic Number ☼Number of protons in an atom ☼Smaller number on the periodic table (p. t. ) 6 Atom mber u N ic C Carbon 12. 011 Element Symbol Element Name
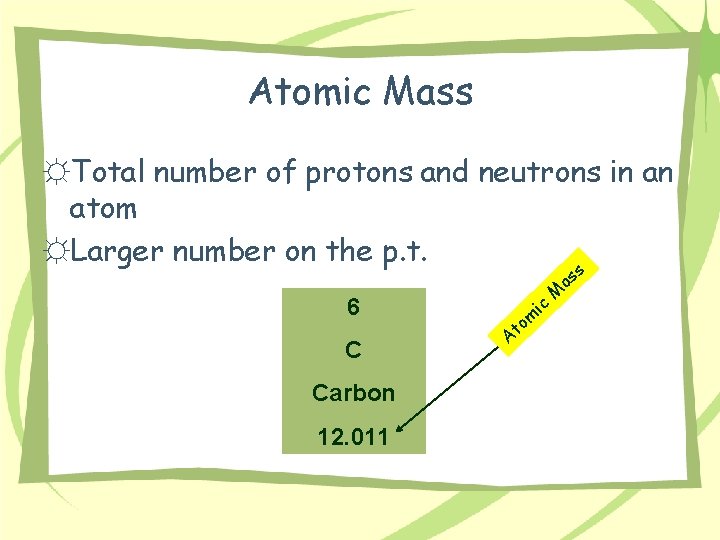
Atomic Mass ☼Total number of protons and neutrons in an atom ☼Larger number on the p. t. 6 C Carbon 12. 011 t A ic m o M as s
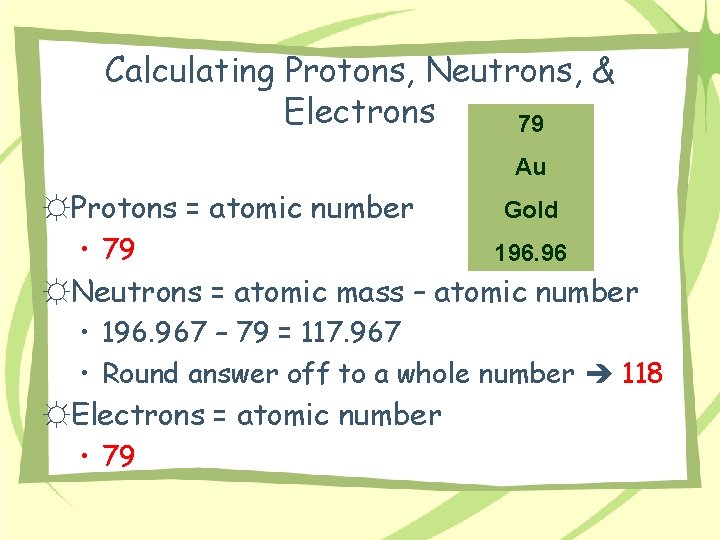
Calculating Protons, Neutrons, & Electrons 79 Au ☼Protons = atomic number • 79 Gold 196. 96 ☼Neutrons = atomic mass – atomic number • 196. 967 – 79 = 117. 967 • Round answer off to a whole number 118 ☼Electrons = atomic number • 79
Summary • Take a few minutes to sum up the notes you have just taken!
- Slides: 11