Unit 1 The Building Blocks of Chemistry Section
Unit 1: The Building Blocks of Chemistry Section 3: Ions
Ions ☼ An atom/molecule that has gained or lost electrons. • Elements on the left of the p. t. “zigzag” are “losers” • Elements on the right of the p. t. “zigzag” are “gainers”
Properties of Ions ☼ Only the number of electrons changes • The number of protons and neutrons do not change

Positive Ions ☼Formed when an atom loses one or more electrons • Protons › electrons, making it positive

Examples of Positive Ions ☼Sodium • Na 1+ +11 -11 -10 ø 12 +13 -13 -10 ø 14 ☼Aluminum • Al 3+

Practice Problems Bring out your periodic table!
Negative Ions ☼Formed when a neutral atom gains one or more electrons • Electrons › protons, making it negative
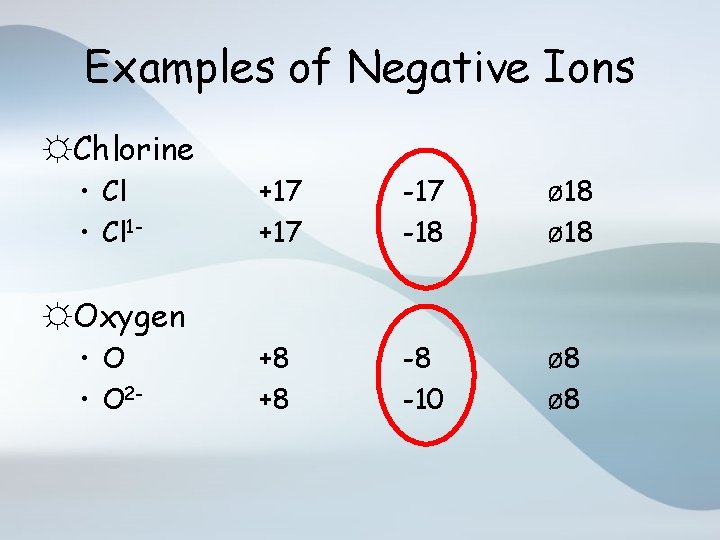
Examples of Negative Ions ☼Chlorine • Cl 1 - +17 -17 -18 ø 18 +8 +8 -8 -10 ø 8 ☼Oxygen • O 2 -

Practice Problems Bring out your periodic table!
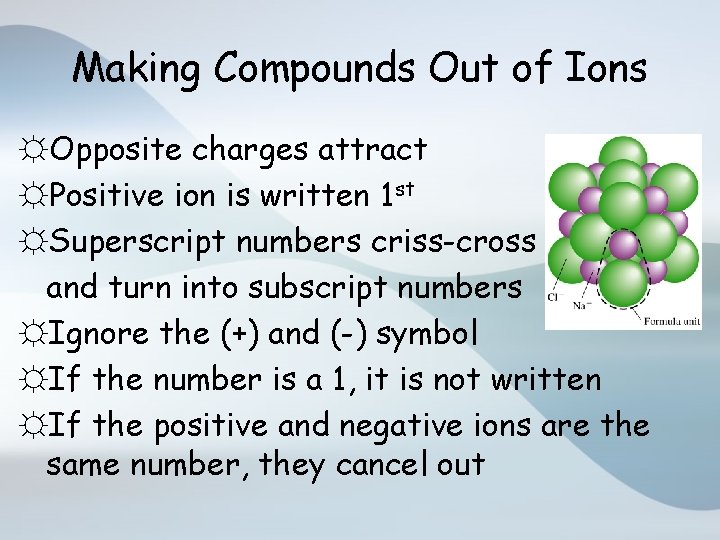
Making Compounds Out of Ions ☼Opposite charges attract ☼Positive ion is written 1 st ☼Superscript numbers criss-cross and turn into subscript numbers ☼Ignore the (+) and (-) symbol ☼If the number is a 1, it is not written ☼If the positive and negative ions are the same number, they cancel out
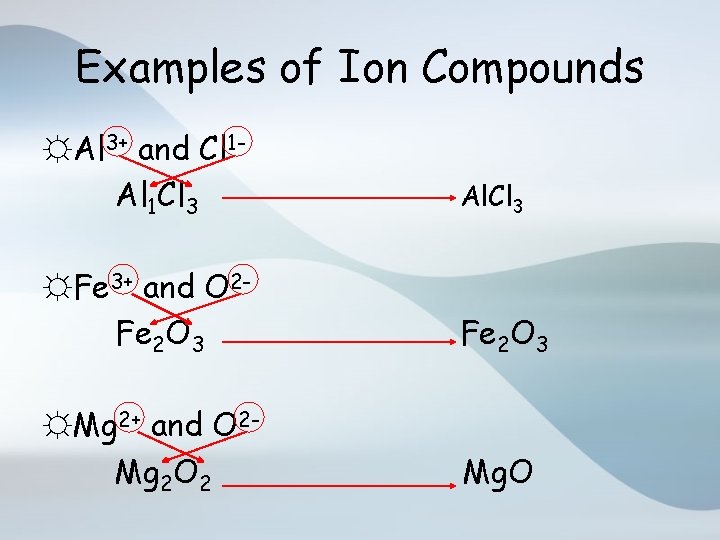
Examples of Ion Compounds ☼Al 3+ and Cl 1 Al 1 Cl 3 Al. Cl 3 ☼Fe 3+ and O 2 Fe 2 O 3 ☼Mg 2+ and O 2 Mg 2 O 2 Mg. O

Practice Problems
- Slides: 12