Unit 1 Structure and Properties of Matter What













































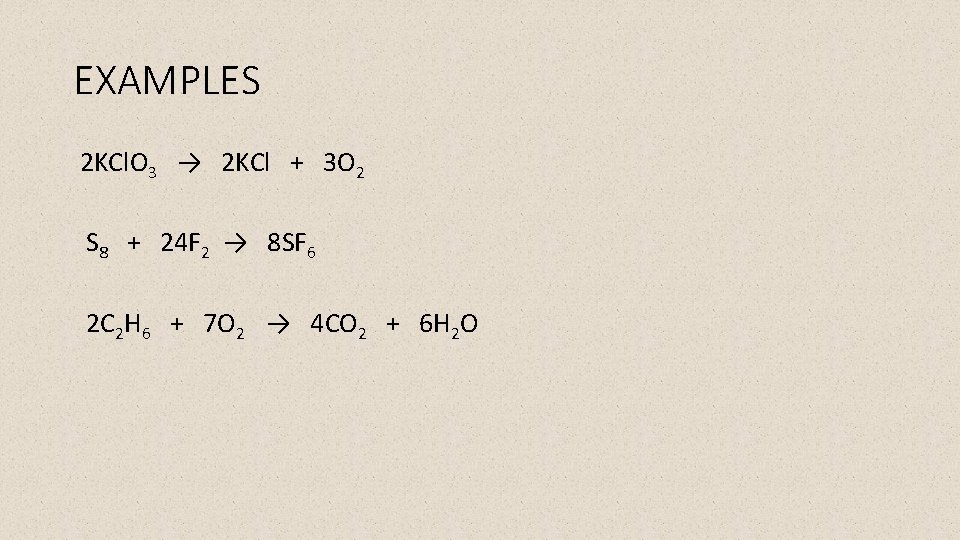





- Slides: 81

Unit 1 Structure and Properties of Matter

What is Matter? - Anything that has mass and takes up space (volume). - Matter describes all of the physical substances around us: your table, your body, a pencil, water, and so forth

Matter • Includes all things that can be seen, tasted, smelled, or touched • Does NOT include heat, sound, or light.


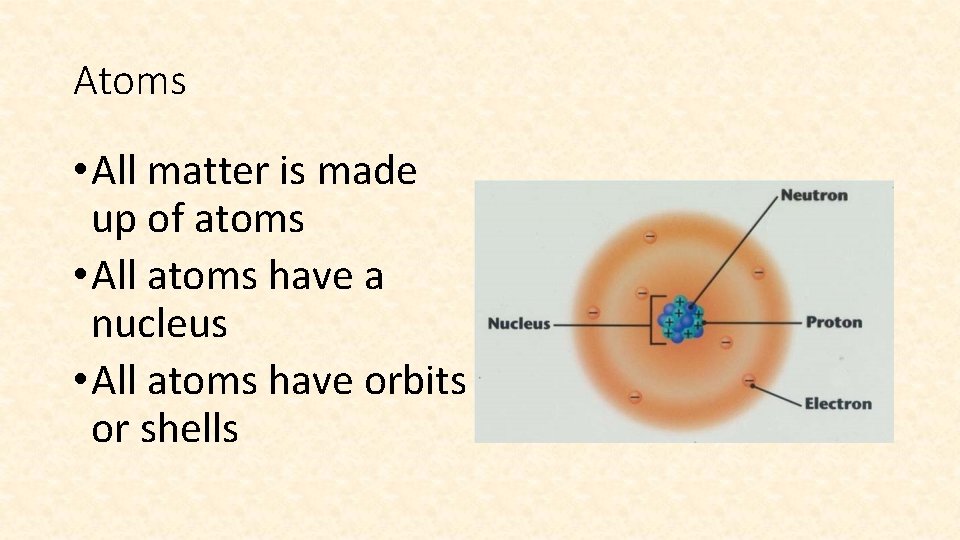
Atoms • All matter is made up of atoms • All atoms have a nucleus • All atoms have orbits or shells

What is in the Nucleus? • Protons (SUBATOMIC PARTICLE) • Protons have a positive charge (+) • The number of protons tells us what kind of element it is!!!

What is in the Nucleus? • Neutrons (SUBATOMIC PARTICLE) • Large and heavy like protons • Neutrons have no electrical charge

What is NOT in the Nucleus? • Electrons • Tiny, very light particles • Have a negative electrical charge (-) • Randomly orbit the nucleus

How do we know what atoms look like? Models and the History of the Atom.

Models • Models are often used for things that are too small or too large to be observed or that are too difficult to be understood easily • Atoms are very small

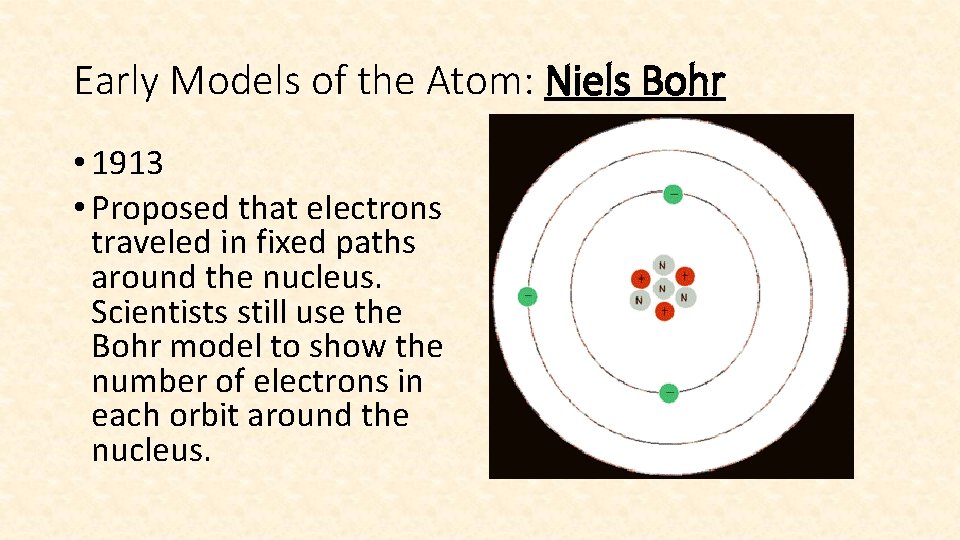
Early Models of the Atom: Niels Bohr • 1913 • Proposed that electrons traveled in fixed paths around the nucleus. Scientists still use the Bohr model to show the number of electrons in each orbit around the nucleus.

Let’s REVIEW!

LET’S REVIEW… • Elements are made up of only one type of atom. • If I took a piece of aluminum foil and ripped it into the tiniest pieces possible, the foil would still have the properties of Aluminum. • The atom is the smallest part of an element that retains the properties of the element.

Atoms Review • Have three particles inside them. • They are known as subatomic particles. • Those are… • Protons • Neutrons • Electrons Let’s meet the Atoms Family https: //www. youtube. com/watch? v=D-i. PPw. DAk 1 Q

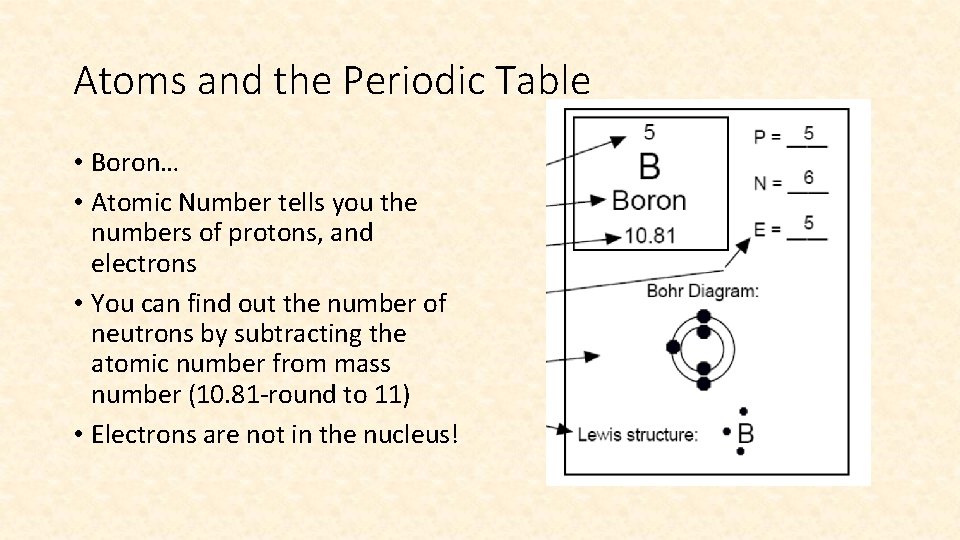
Atoms and the Periodic Table • Boron… • Atomic Number tells you the numbers of protons, and electrons • You can find out the number of neutrons by subtracting the atomic number from mass number (10. 81 -round to 11) • Electrons are not in the nucleus!

One more time… • For any element: • Number of Protons = Atomic Number • Number of Electrons = Number of Protons • Number of Neutrons = Mass Number - Atomic Number

BELL WORK • Determine the number of protons, number of electrons, and number of neutrons for the first ten elements on the Periodic Table of Elements.

Using How To Bohr Diagram ppt (separate)

Sharing makes you happy! • Some atoms have too many valence electrons (# of electrons in the outer most shell/ orbit) • Some atoms have too little valence electrons. • Everyone wants a FULL valence shell 1 st = 2 electrons 2 nd= 8 electrons 3 rd= 8 electrons Sooooooo… They will join forces in order to make sure that the other atom is HAPPY. This makes a MOLECULE!

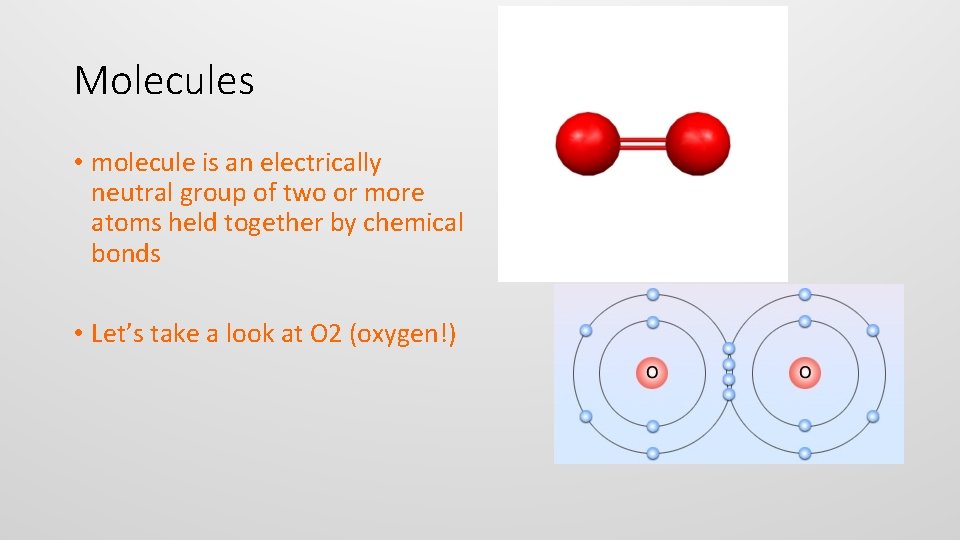
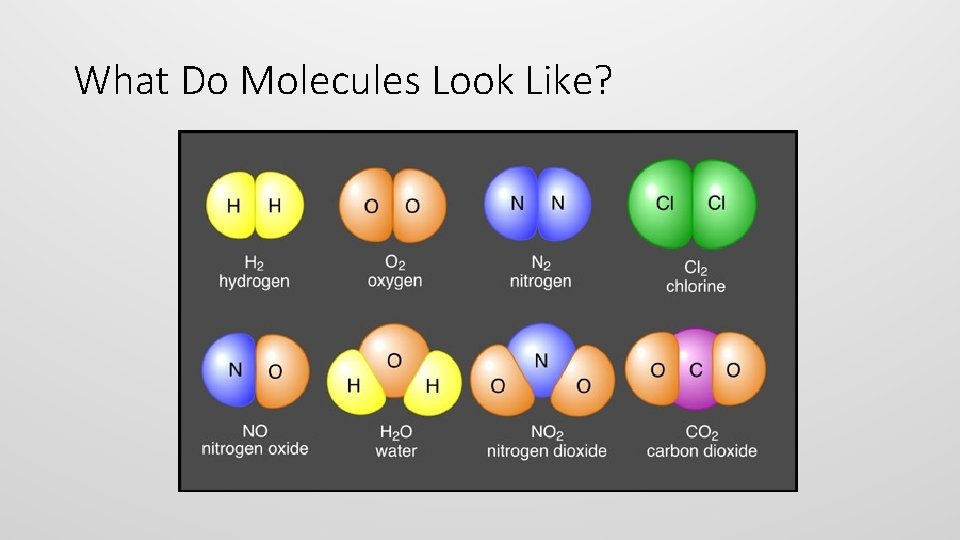
Molecules • molecule is an electrically neutral group of two or more atoms held together by chemical bonds • Let’s take a look at O 2 (oxygen!)

Two Types of Molecules • Molecules of an Element • Molecules of a Compound • Molecules that contain a single element but multiple atoms. • A compound is a molecule that contains at least two different elements. • All compounds are molecules but not all molecules are compounds. • E. g. Oxygen (O 2), Hydrogen (H 2), and Nitrogen (N 2)

What Do Molecules Look Like?

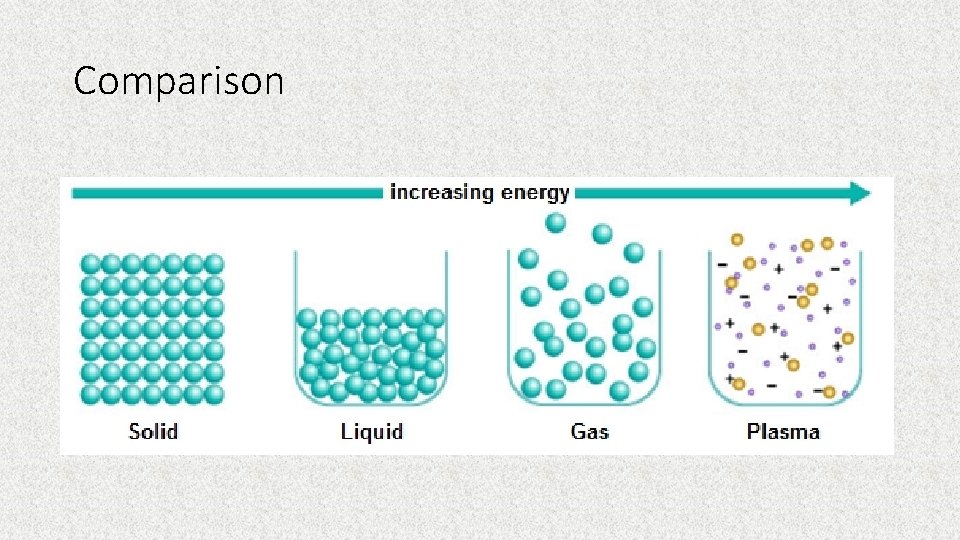
STATES OF MATTER SOLID LIQUID GAS PLASMA

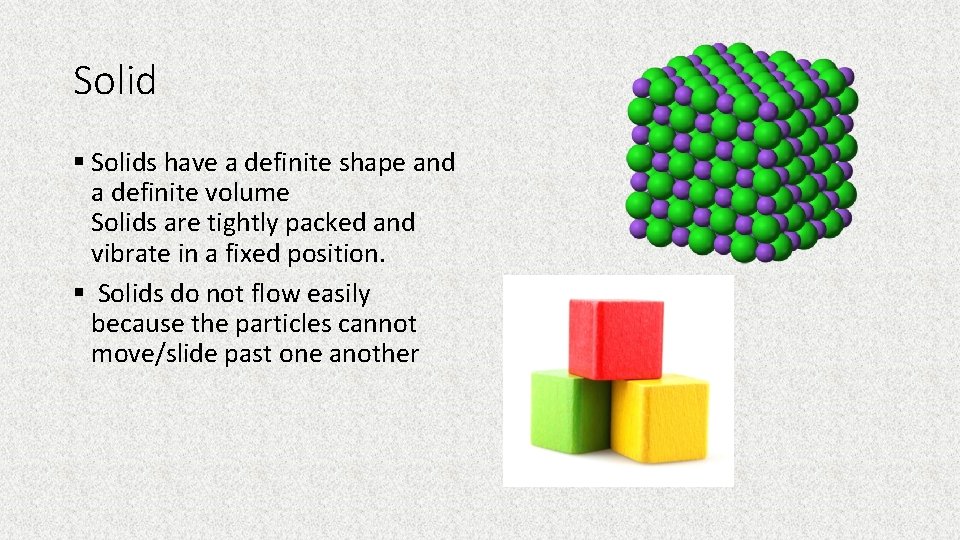
Solid § Solids have a definite shape and a definite volume Solids are tightly packed and vibrate in a fixed position. § Solids do not flow easily because the particles cannot move/slide past one another

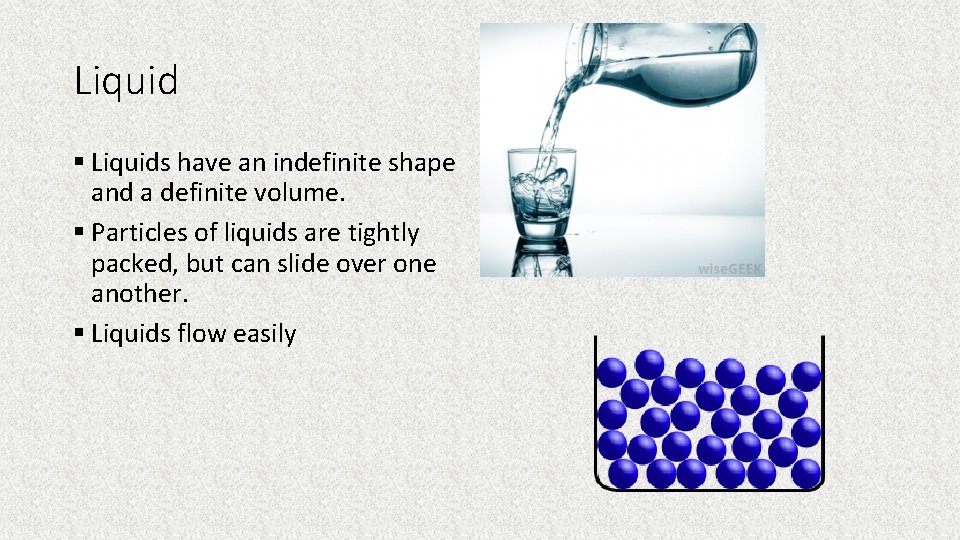
Liquid § Liquids have an indefinite shape and a definite volume. § Particles of liquids are tightly packed, but can slide over one another. § Liquids flow easily

Gas § Gases have an indefinite shape and an indefinite volume. § Particles of gases are very far apart and move freely § Gases flow very easily

Plasma § Plasma, like gases have an indefinite shape and an indefinite volume § A plasma is a charged gas. § A plasma is a very good conductor of electricity and is affected by magnetic fields

Comparison

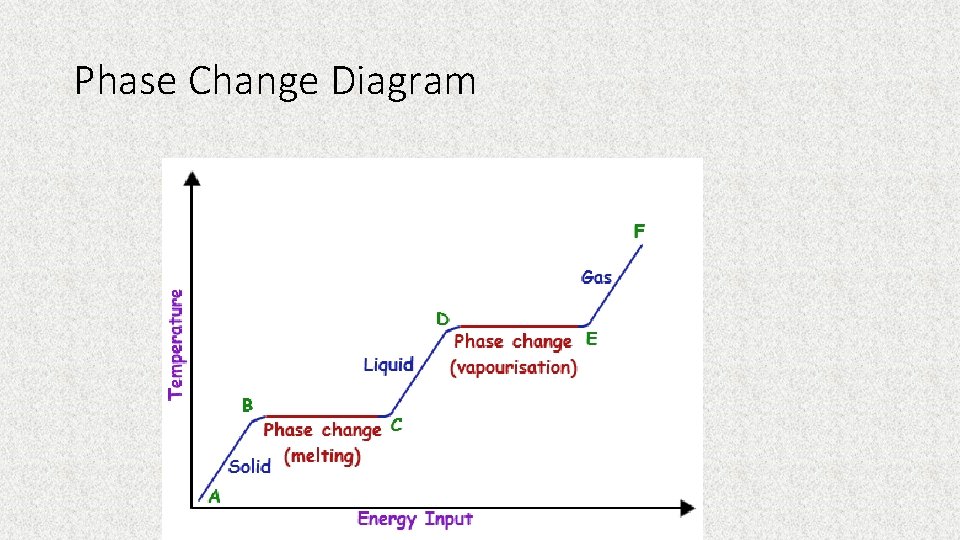
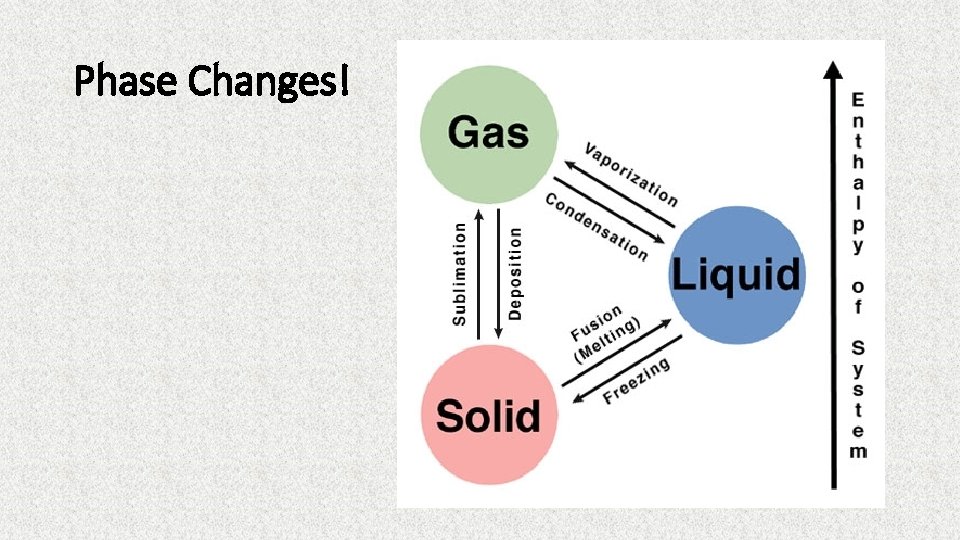
What are some ways we can add or take away energy from a system?

Adding or Taking Away Energy • Shaking • Stirring (sweet tea) • Adding or removing heat

Phase Change Diagram

Phase Changes!

PHYSICAL AND CHEMICAL PROPERTIES OF MATTER

Physical Properties of Matter • Anything that can be observed or measured without changing the identity.

Physical Properties Examples • Density (space between particles) • State (solid, liquid, gas, plasma) • Solubility ( can it dissolve in water) • Conductivity( does it allow heat or electricity to pass through)

Physical Properties Examples, cont’d • Ductility (can it pulled out into thin strips like wires) • Malleability ( can it be pounded into flat sheets like aluminum) • Color, shape, size, mass, volume, texture, smell etc.

Chemical Properties of Matter • Describes how easily a piece of matter can change into something new. • YOU CANNOT OBSERVE A CHEMICAL PROPERTY WITHOUT CHANGING THE MATTER.

Chemical Properties Examples • Flammability (easily catches fire ) • Reactivity ( does it interact with other substances easily) • Combustibility ( will it burn) More vigorous conditions are required for an ideal combustible material to burn

Physical Change • Any change that changes the appearance but NOT the identity of the substance Examples: Melting Ice ( or any change of state) Flattening metal with a hammer Dissolving sugar in water Slicing watermelon Ability to transfer heat/electricity

Chemical Change • Any change that changes the identity. The matter changes into something NEW with new physical and chemical properties Examples Burning wood Fireworks exploding Rotting food or souring milk Rusting

How do you know you’ve had a PHYSICAL CHANGE? • Texture - Rough and finished wood • Color - Painting a car • Temperature - Pan heating up • Shape - orgami • Change of State

How do you know you’ve had a CHEMICAL change? • Change in Temperature - Sodium in water video • Change in Color - Rust metal • Noticeable Odor (after reaction has begun) - Spoiled egg • Formation of a Precipitate - Silver extraction video. Silver precipitated on the copper wire. • Formation of Bubbles -Hydrogen gases created with sodium in water

Periodic Table and Elements

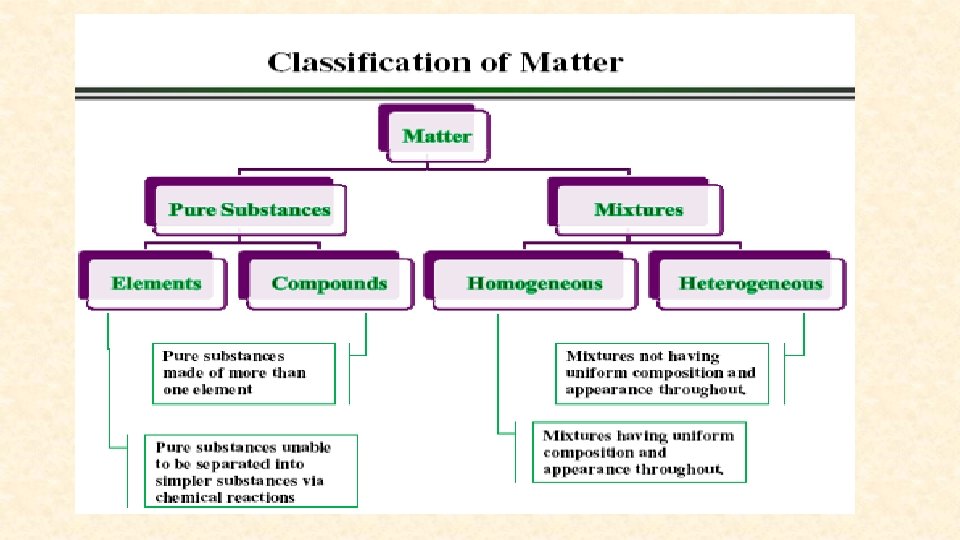
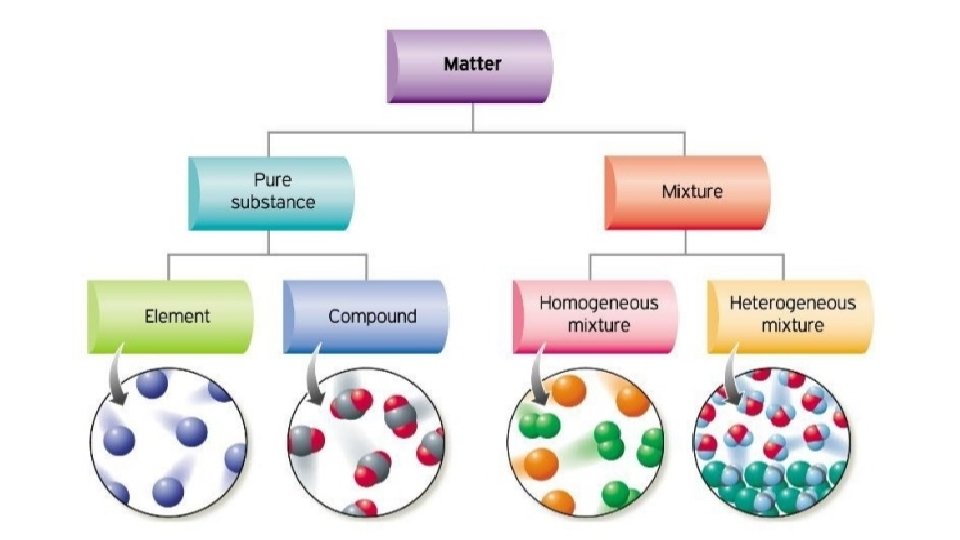
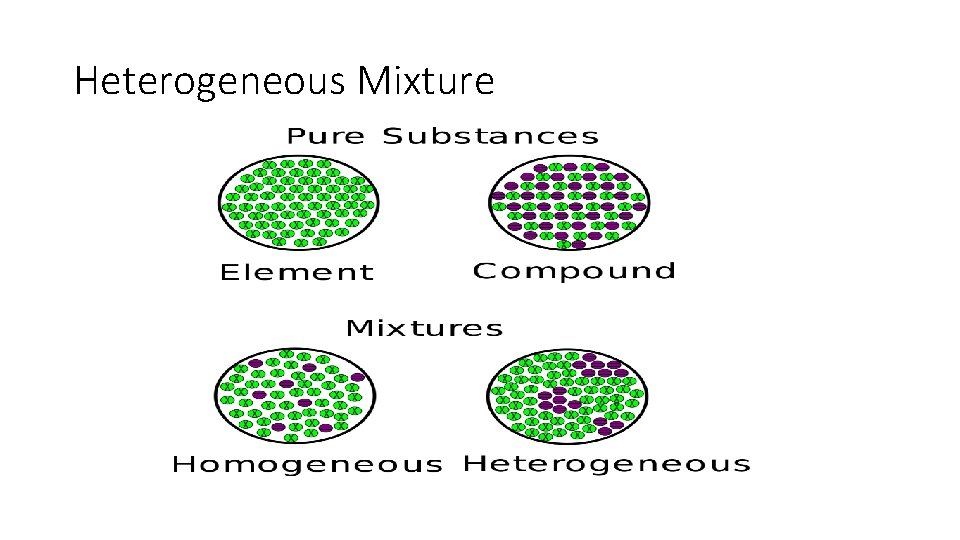
Elements 1. AN ELEMENT IS A SUBSTANCE THAT CANNOT BE BROKEN DOWN INTO SIMILAR SUBSTANCES BY PHYSICAL OR CHEMICAL MEANS. 2. AN ELEMENT IS A PURE SUBSTANCE 3. ELEMENTS CAN BE IDENTIFIED BY THEIR CHARACTERISTIC PROPERTIES

Pure Substance Definition • A PURE SUBSTANCE IS A SUBSTANCE HAS PARTICLES THAT ARE ALL THE SAME AND THEY HAVE UNIQUE PROPERTIES.

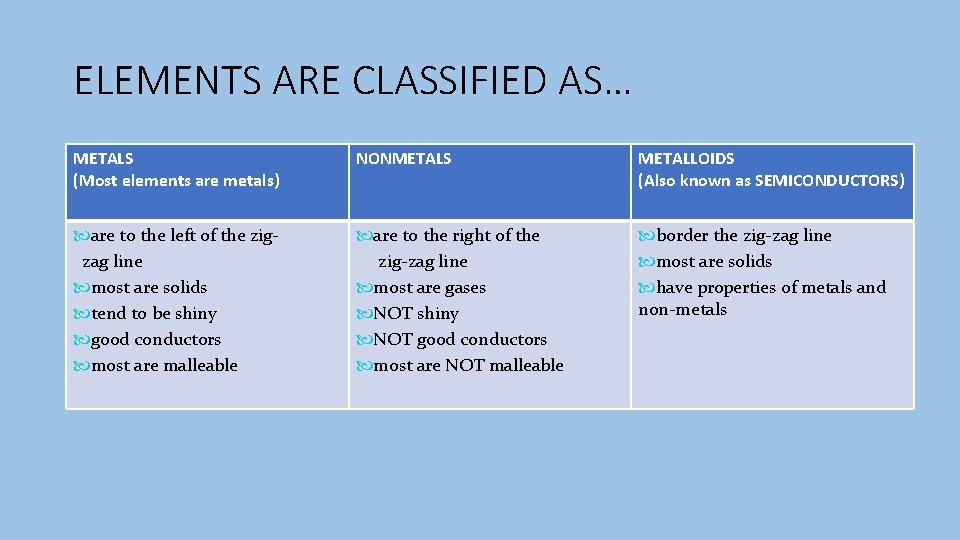
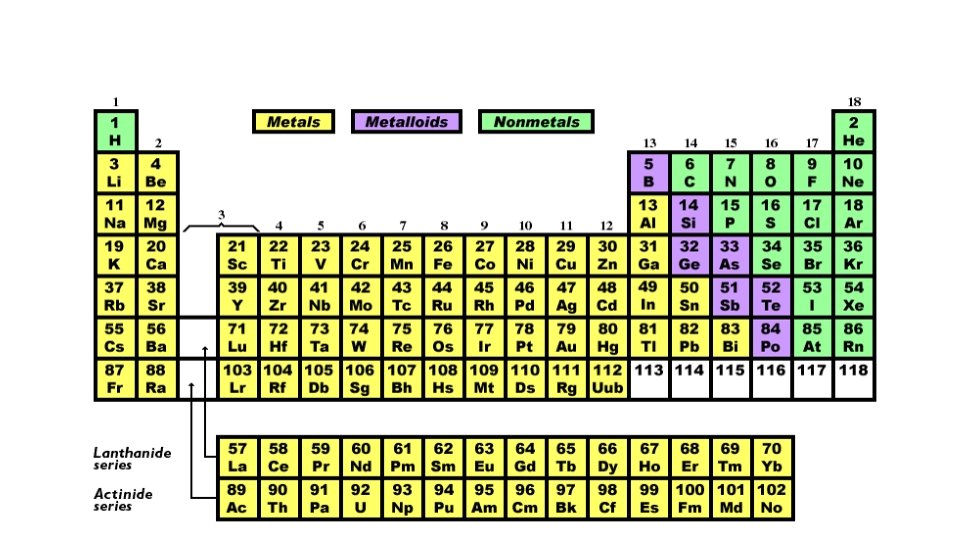
ELEMENTS ARE CLASSIFIED AS… METALS (Most elements are metals) NONMETALS METALLOIDS (Also known as SEMICONDUCTORS) are to the left of the zigzag line most are solids tend to be shiny good conductors most are malleable are to the right of the zig-zag line most are gases NOT shiny NOT good conductors most are NOT malleable border the zig-zag line most are solids have properties of metals and non-metals

Mendeleev • Number elements according to atomic mass. • Noticed that properties were repeated in a regular pattern. • That is why it is called the PERIODIC table.

PROBLEM: A Few Elements Did Not Fit. • So, a British Scientist came along and reordered them according to atomic number. • They found that elements fit into a pattern.


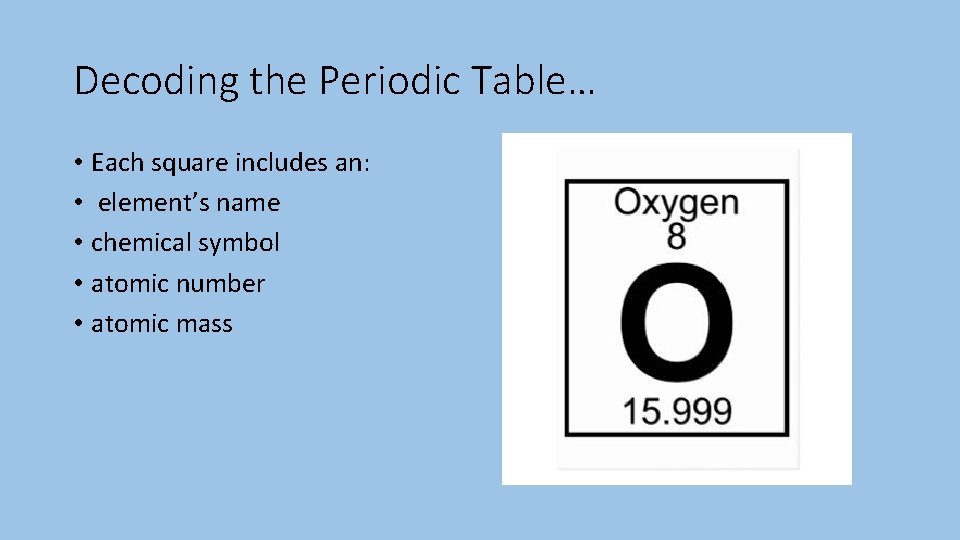
Decoding the Periodic Table… • Each square includes an: • element’s name • chemical symbol • atomic number • atomic mass

Decoding the Periodic Table • Rows are called PERIODS. As you move across a row, the properties change gradually Example: very metallic-Ti to less metallic-Ge to non-metallic-Br • Columns are called GROUPS. Elements in the same group have similar physical and chemical properties. For this reason, a group is also called a FAMILY

COMPOUNDS AND MIXTURES!

Play this game! • http: //bbc. in/1 e. Ahq. XQ

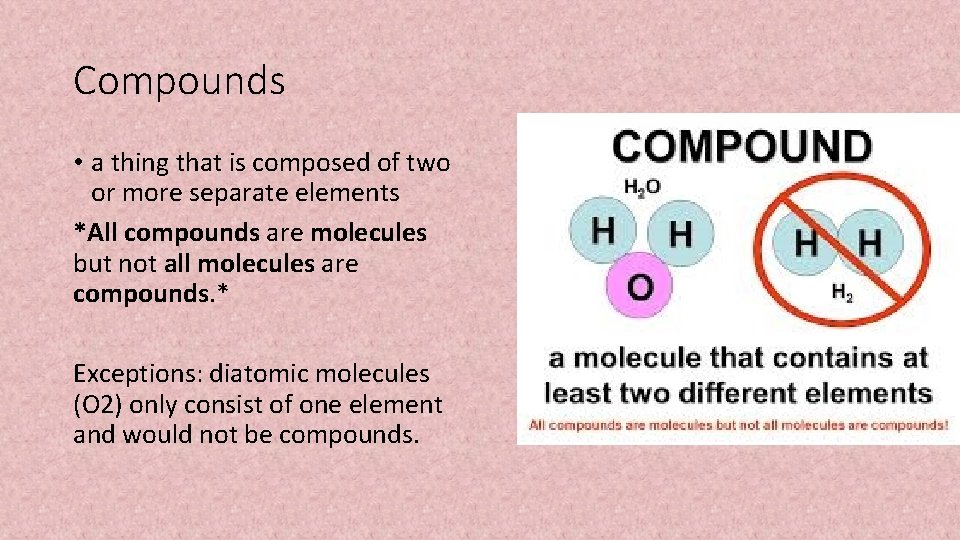
Compounds • a thing that is composed of two or more separate elements *All compounds are molecules but not all molecules are compounds. * Exceptions: diatomic molecules (O 2) only consist of one element and would not be compounds.

MIXTURES • Homogenous • Heterogenous simply any mixture that is uniform Any mixture where substances are in composition throughout. not mixed on a molecular level NOT UNIFORM! Examples: milk, kool-aid, blood, lotion, window cleaner, glue. Examples: pizza, chocolate chip cookie, Twix.



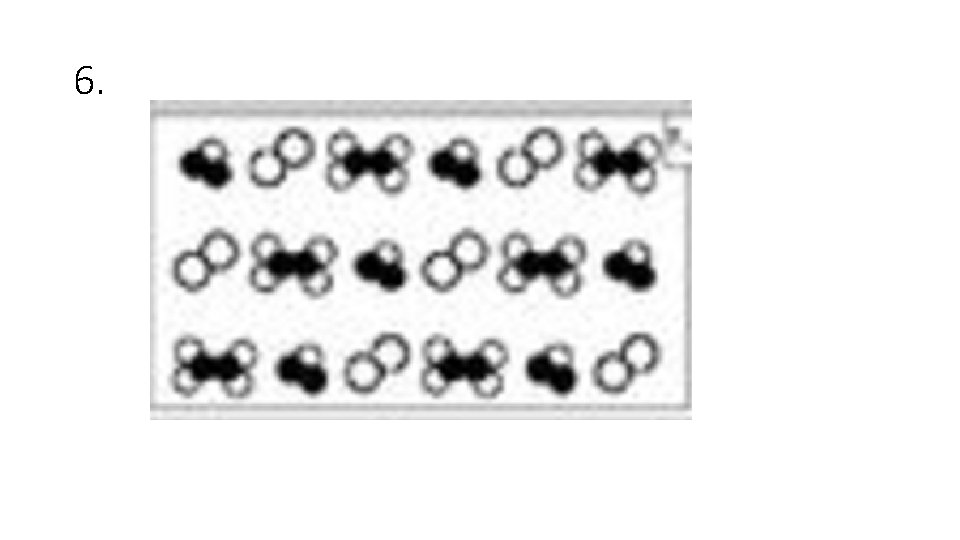
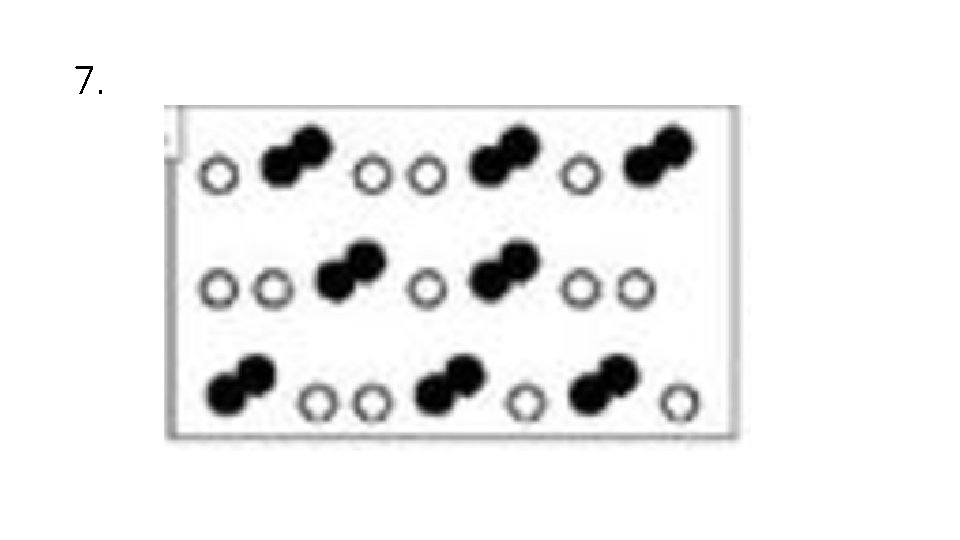
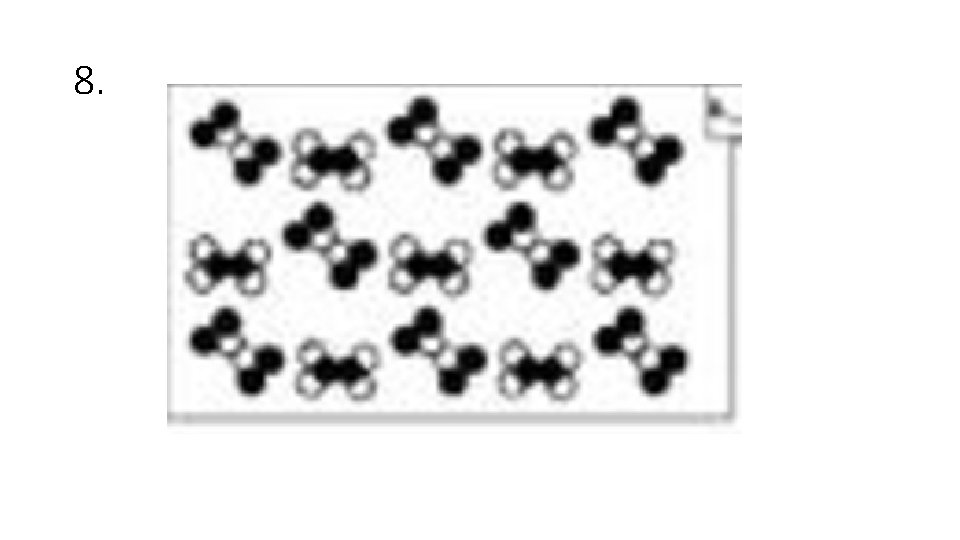
Answer Choices A. Elements B. Compounds C. Mixtures of Elements D. Mixtures of Compounds E. Mixture of Elements and Compounds

1.

2.

3.

4.

5.

6.

7.

8.

Heterogeneous Mixture

Conservation of Mass

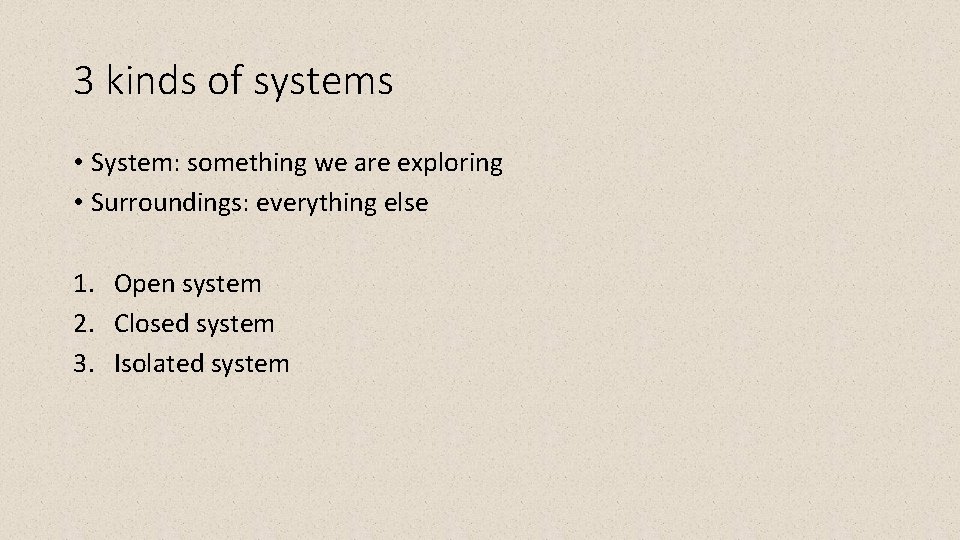
3 kinds of systems • System: something we are exploring • Surroundings: everything else 1. Open system 2. Closed system 3. Isolated system

Open System • Open: Energy AND matter can flow in and out of the system with ease! • E. g. Boiling pot of water on the stove.

Closed System • Energy can flow in and out BUT matter does NOT flow in and out. • E. g. boiling water with lid.

Isolated System • NO energy NOR matter flowing in and out of the system. • E. g. insulated container.

Law of Conservation of Mass • states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. • The mass of the products in a chemical reaction must equal the mass of the reactants.

Reactions 2 Zn + 4 HCl → 2 Zn. Cl 2 + 2 H 2 Where are the reactants? Where are the products? If I react 3 g of Zinc and 5 g of Hydrochloric acid in an isolated system how many grams of zinc chloride and hydrogen gas will I end up with?
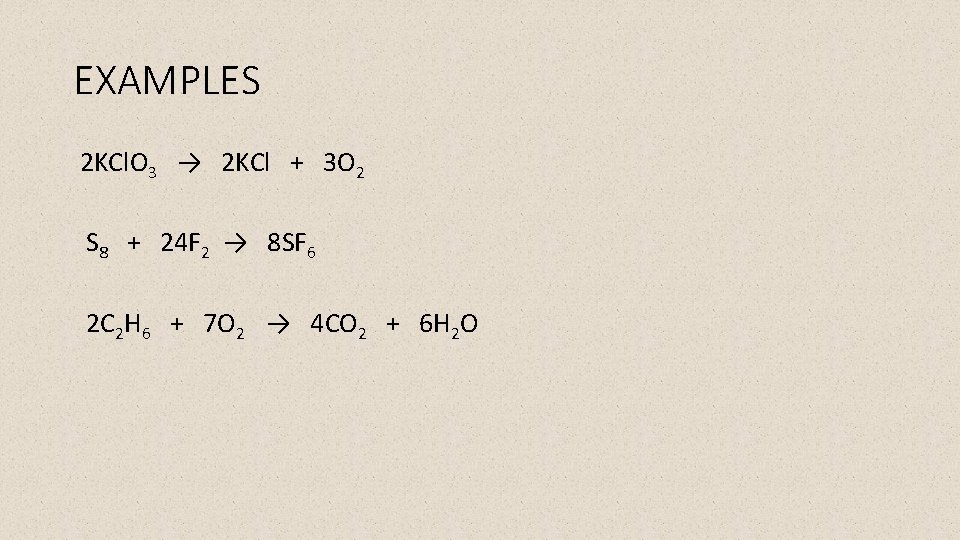
EXAMPLES 2 KCl. O 3 → 2 KCl + 3 O 2 S 8 + 24 F 2 → 8 SF 6 2 C 2 H 6 + 7 O 2 → 4 CO 2 + 6 H 2 O

Lab Tomorrow •

Density the degree of compactness of a substance

Density of Solids. • Would you rather carry around a pound of feathers or a pound of lead?

Answer • At first you may think feathers… but one pound of each will be the same amount of weight. However, the feathers are much less dense so it will take a lot more feathers to equal one pound. • This makes lead the better answer!

Density • Density can tell you whether a substance will float or sink. • IF the density of an object is LESS than the density of water it will float. • IF the density of an object is MORE than the density of water it will sink.

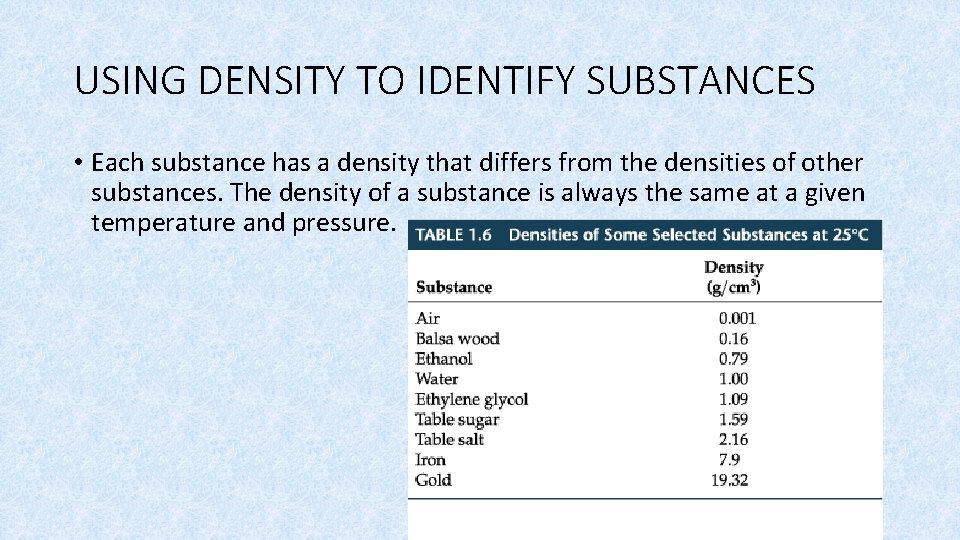
USING DENSITY TO IDENTIFY SUBSTANCES • Each substance has a density that differs from the densities of other substances. The density of a substance is always the same at a given temperature and pressure.