Unit 1 Section 1 Introduction to Chemistry CHEMISTRY





































- Slides: 44

Unit 1 Section 1 - Introduction to Chemistry

CHEMISTRY n & YOU What is this creature? • Fugu, also known as puffer fish, is a sushi delicacy that can also be lethal. • Recently this toxin has been put to good use, as scientists have discovered that a purified form of it can treat severe pain in cancer patients. http: //www. nbcnews. com/healthnews/frightful-fish-tale-doctors-warn-poisonpufferfish-n 277591

Topics Chemistry and its Branches n Scientific Method n Green Chemistry n

Objectives n n n Define Chemistry Differentiate between the 5 branches of Chemistry Apply the general plan in solving everyday problems using the scientific method

n Matter – anything that takes up space and has mass n Chemistry – the study of matter and the changes that matter undergoes Vocabulary n Organic Chemistry – the study of all chemicals that contain carbon Matter -Chemistry -Organic chemistry -Inorganic Chemistry -Biochemistry -Analytical Chemistry -Physical Chemistry -Pure Chemistry -Applied Chemistry -Technology n Inorganic Chemistry – the study of chemical that do not contain carbon n Biochemistry – the study of processes that take place in organisms n Analytical Chemistry – the study of composition of matter n Physical Chemistry – the area that deals with the mechanism, the rate and the energy transfer that occurs when matter undergoes a change 1. 1 Chemistry -

What Is Chemistry? Chemistry answers many questions you may have about the world you live in. • Chemistry is the study of the composition of matter and the changes that matter undergoes. Video – What is Chemistry?

What Is Chemistry? Matter is anything that has mass and occupies space. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

https: //www. youtube. com/watch? v=E 1 Tk. Du. GFDdc https: //www. youtube. com/watch? v=66 SGc. BAs 04 w

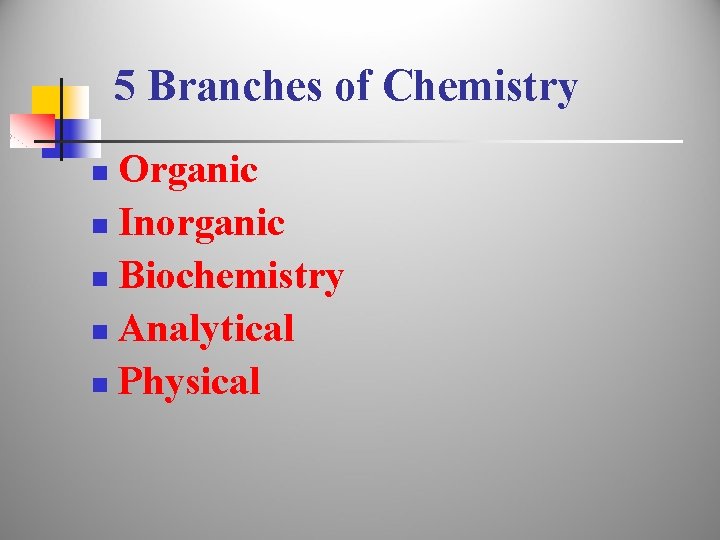
5 Branches of Chemistry Organic n Inorganic n Biochemistry n Analytical n Physical n

n Organic Chemistry – the study of all chemicals that contain carbon o examples: CO 2 , Carbon dioxide n C 6 H 12 O 6 Glucose Inorganic Chemistry – the study of chemicals that do not contain carbon o examples: H 2 O water , Na. Cl salt (sodium chloride)

n Biochemistry – the study of chemical processes that take place in organisms examples: photosynthesis, cellular respiration o n Analytical Chemistry – the study of composition of matter o example: determining the composition of a penny q q % of copper % of zinc

n o Physical Chemistry – deals with the rate and the energy transfer that occurs when matter undergoes a change example: the rate at which gasoline burns

Identify some of the components of this picture and match it with one of the Chemistry branches

QUIZLET 1) Click Test 2) Select only Written

Read each goal and complete the table by filling in one of the five main areas of chemistry. Branch of Chemistry Goals Developing a new carbon-based fiber for clothing Developing a better insulin-delivery system for diabetics Determining the amount of mercury present in a soil sample Comparing the hardness of copper and silver Investigating ways to slow down the rusting of steel

Chemistry: In the making n Producing aspirin tablets so that consumers can use them.

1. 2 Energy Chemists play an essential role in finding ways to conserve energy, produce energy, and store energy. n DO NOW 1. How is energy produced by chemists? List some types of energy produced. 2. How do people conserve energy?

GREEN CHEMISTRY n Green chemistry is the science of creating safe, energy efficient and non-toxic products and processes in order to solve environmental problems

What areas do environmental issues affect? n Three areas: n n n air quality land quality water quality

Air Quality Why is air quality such a problem? Poor air quality can lead to: n smog n respiratory & other illnesses n acid rain n global warming

Greenhouse Gases & Global Warming • • • Global warming: An increase in the average air temperature of the Earth. Greenhouse effect: Heat from the sun gets trapped inside the glass of a greenhouse and heats up its air. More carbon dioxide (CO 2) being released in the atmosphere traps more heat.

Environmental issues What causes: air pollution? land pollution? water pollution?

Examples Renewable Resources Non-Renewable Resources

Non-renewable Energy Sources Limited supply of resources n Fossil fuels n o o o Coal Natural gas Oil

Renewable Energy Resources n n Hydroelectric power – flowing water creates energy that can be captured and turned into electricity. Solar power – produced by collecting sunlight and converting it into electricity. Biomass – fuel developed from biological material derived from living and nonliving organisms Geothermal energy – is heat from the earth that can create clean energy

Production of Energy n Hydroelectric plants use running water to generate electricity, however they may flood nearby lands and can disrupt the normal flow of water, both of which negatively affect the environment.

n n Wind power is increasingly being used as a clean source of renewable energy. Turbines harvest wind on wind farms and generate electricity.

n Solar power is a promising, renewable energy resource than can be turned into electricity, and it is used in many toys and even home heating.

Many other alternative energy sources like: -geothermal power, which draws upon the earth’s natural heat, and - biomass, which produces an alternative to gasoline, are being considered in the movement away from fossil fuel dependence. n

Conservation of Energy n In our everyday lives, we can also work to conserve energy. n Insulating, turning off lights and only using appliances like dishwashers when they are full are just some of the ways people can limit energy use in their homes. n Also, carpooling, bicycling, and taking public transportation are effective energy-saving ideas.

Scientific Method Activity

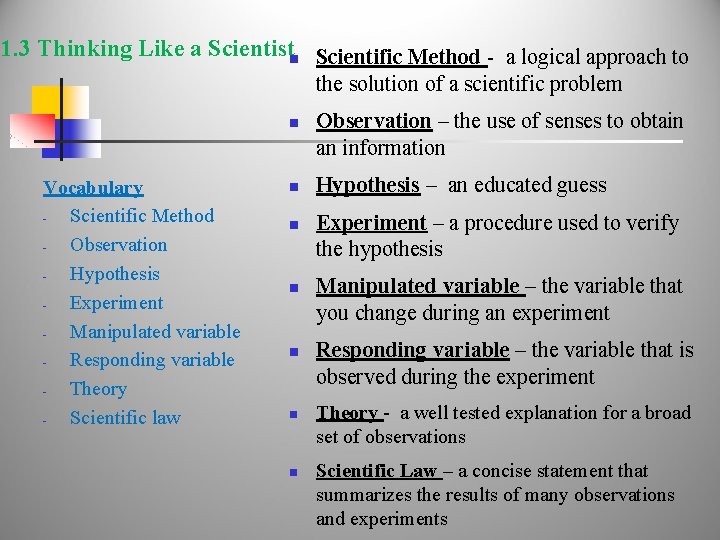
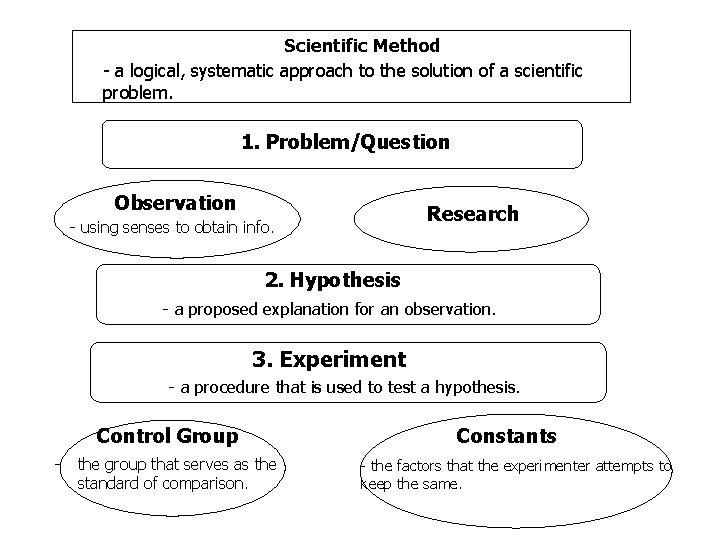
1. 3 Thinking Like a Scientistn Scientific Method - a logical approach to the solution of a scientific problem n Vocabulary Scientific Method Observation Hypothesis Experiment Manipulated variable Responding variable Theory Scientific law n n n Observation – the use of senses to obtain an information Hypothesis – an educated guess Experiment – a procedure used to verify the hypothesis Manipulated variable – the variable that you change during an experiment Responding variable – the variable that is observed during the experiment Theory - a well tested explanation for a broad set of observations Scientific Law – a concise statement that summarizes the results of many observations and experiments

n Problem solving is a skill you use all the time. n n For example, a shopper must make many decisions. Some of those are based on data, like the information on a food label. The skills you use to solve a word problem in chemistry are not that different from those you use while shopping.

Scientific Method - a logical, systematic approach to the solution of a scientific problem. 1. Problem/Question Observation Research - using senses to obtain info. 2. Hypothesis - a proposed explanation for an observation. 3. Experiment - a procedure that is used to test a hypothesis. Control Group - the group that serves as the standard of comparison. Constants - the factors that the experimenter attempts to keep the same.

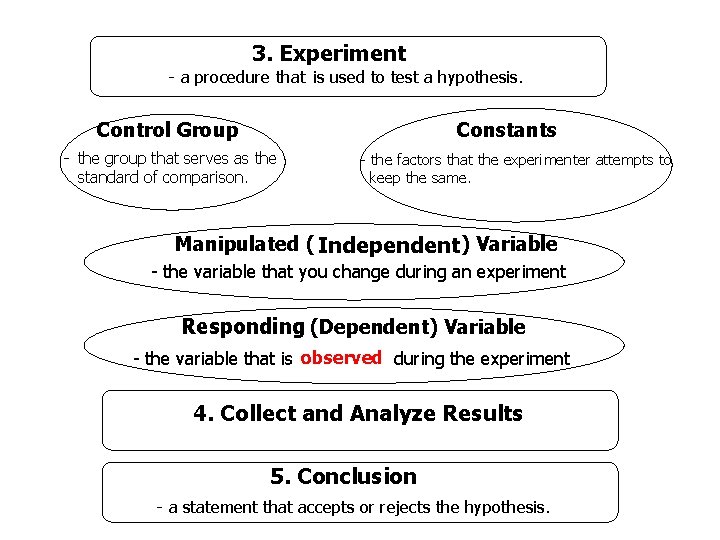
3. Experiment - a procedure that is used to test a hypothesis. Control Group Constants - the group that serves as the standard of comparison. - the factors that the experimenter attempts to keep the same. Manipulated ( Independent) Variable - the variable that you change during an experiment Responding (Dependent) Variable - the variable that is observed during the experiment 4. Collect and Analyze Results 5. Conclusion - a statement that accepts or rejects the hypothesis.

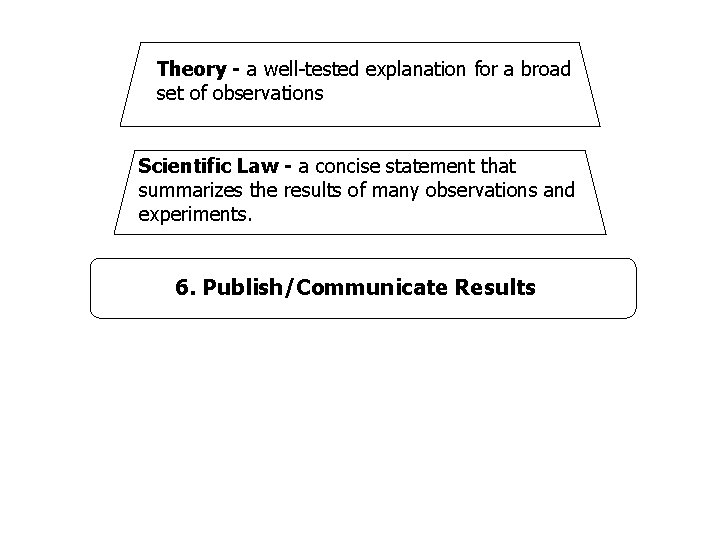
Theory - a well-tested explanation for a broad set of observations Scientific Law - a concise statement that summarizes the results of many observations and experiments. 6. Publish/Communicate Results

Scientific Method n n Definition – A logical, systematic approach to solving problems. Steps of the Scientific Method 1. Problem/Question 2. Hypothesis 3. Experiment 4. Collect and Analyze Results 5. Conclusion 6. Publish Results (Communicate the Results)

Steps of the Scientific Method 1. Problem/Question – a question to be answered 2. Hypothesis – a proposed explanation for an observation n Make observations – using senses n Research your topic of interest.

3. Experiment – a procedure used to test a hypothesis. n Control - the group that serves as the standard of comparison n Manipulated variable – a variable you change n Responding variable – a variable you observe n Constant - all the factors that the experimenter attempts to keep the same

4. Collect and Analyze Results - Include tables, graphs, and photographs. 5. Conclusion – analyze and summarize experimental results to form theories or laws. n n Theory – a well tested explanation for a broad set of observations Scientific Law – a concise statement that summarizes the results of many observations and experiments

6. Communicate the Results - Be prepared to share with an audience - Expect questions from the audience.

Theory n n A well tested explanation for a broad set of observations. May use models. May allow predictions. Theories may change to explain new observations.

Law n n A statement that summarizes results of observations, but does not explain them. Changes or is abandoned when contradicted by new experiments.

Note: n The order of the steps can vary and additional steps may be added.