Unit 1 Scientific Method Graphs Metric System and



































- Slides: 41

Unit 1 Scientific Method, Graphs, Metric System, and Measurement

Physical Science l Physical science is the study of l Matter, which is also known as chemistry l. Matter is anything that takes up space l. Like animals, people, rocks, and air l Energy, which is also known as physics l. Energy is the ability to cause change l. Like lightning, motion, and sound

What We Know in Science A fact is something known to be true. Facts can be gained by observation, or using your senses. l A theory is a logical explanation for events which occur in nature. l A scientific law is a theory that has been tested many times and is generally accepted as true. l

Scientific Method l l How do scientists investigate questions? The scientific method is a system that scientists use to solve problems

Scientific Method l The scientific method includes 7 steps 1. 2. 3. 4. 5. 6. 7. Stating the problem Gathering information on the problem Forming a hypothesis Performing experiments to test the hypothesis Recording and analyzing data Stating a conclusion Repeating the work

Scientific Method Activity 1. 2. 3. Work with the person next to you. If you are at a table by yourself, work with the pair at the table joining yours. Get 1 bag of m&m’s per group. (DO NOT OPEN BAG YET) Decide on one question you would like to answer about your bag of m&m’s. Write it down. (DO NOT OPEN BAG YET)

How many m&m’s are in a bag? 4. Guess what your answer might be (hypothesize). l Hypothesis: A testable prediction used to see how something works or to solve a problem (Glencoe Physical Science, p. 760)

How do we find out if our hypothesis is correct? 5. Open your bag of m&m’s and count them! l l l Count how many of each color you have, too. Data: Recorded observations and measurements Data collection: Gathering information about our hypotheses. One way to gather data is through observation, which is using your senses We can also gather data by doing an experiment. An experiment is a procedure for testing a hypothesis. (Glencoe Physical Science, p. 758)

Experiments usually have a control, which is a standard to compare your results to. l A constant is a factor that does not change during the experiment. l l In our experiment, the kind of candy we looked at, plain m&m’s, stayed the same

Variables Experiments have variables, which are the factors being tested during the experiment. l Experiments have independent and dependent variables. l Independent variable – A factor that I, as the experimenter, control. l Dependent variable – A factor whose value depends on the value of the independent variable l

Experiments l You can also have multiple trials, in which you look at the same set of variables more than once. In our case, we looked at lots of bags of m&m’s.

What can we say about a big bag of m&m’s? Do we know for sure how many and what colors are in the bag? l Can we make a pretty good guess based on what we know from the small bags? l That is an inference – a conclusion or guess based on evidence. l

My Experiment l What if I wanted to know how your sleep would affect your test grades? l Group 1 – Study 1 hour, sleep 8 hours, arrive to take the test at 8: 00 a. m. l Group 2 – Study 1 hour, sleep 6 hours, arrive to take the test at 8: 00 a. m. l Group 3 – Study 1 hour, sleep 4 hours, arrive to take the test at 8: 00 a. m. l Group 4 – Study 1 hour, sleep 2 hours, arrive to take the test at 8: 00 a. m.

My Experiment What would be a hypothesis for this experiment? Which group might be a good control? Which variable would be my independent variable? Which variable would be my dependent variable? What would be the variables I kept constant? How could I do multiple trials?

How can we look at our data? Use a graph l A graph is a visual display of information or data l

Circle Graphs or Pie Charts l Used to show a fixed quantity is broken down into parts. l The circle represents the total and the slices represent the parts.

Bar Graphs l Useful for comparing information collected by counting. 9 8 7 6 5 Series 1 4 Series 2 Series 3 3 2 1 0 1 2 3 4 5

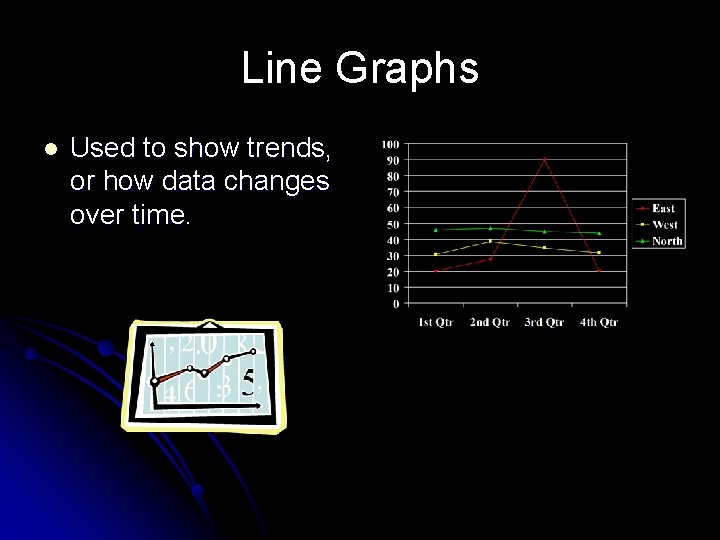
Line Graphs l Used to show trends, or how data changes over time.

Designing an Experiment

The greater the amount of soap in a soap and water mixture, the bigger a soap bubble can be made. l Design an investigation to test this hypothesis. l Identify the variables l What exactly will be changed? How will it be changed? l What exactly will be measured? How will it be measured?

The farther a ball drops, the farther it will bounce. l Design an investigation to test this hypothesis. l Identify the variables l What exactly will be changed? How will it be changed? l What exactly will be measured? How will it be measured?

Measurement l Metric System - System of measurement used by scientists l Also known as the International System of Units, abbreviated SI l Based on the number 10

Length l Length is the distance between two points l The SI base unit is the meter (abbreviated m) l May be measured with a meter stick or a metric ruler

Measuring Length l Start the measurement at the 0 cm mark on the ruler or meter stick l Read the length of the object to the whole centimeter, then count the tenths of a centimeter (or millimeters) and add those in the tenths place, estimate one more digit in the hundredths place

Temperature l Temperature is a measurement of how hot or cold something is l Our thermometers always read ºC l Water freezes at 0ºC l Water boils at 100ºC l The celsius scale is divided into 100 equal divisions l Read thermometer to the nearest degree or half degree

Measuring Temperature l l l Handle thermometers carefully Place them on a paper towel on the table to prevent rolling Do NOT shake Make sure that the bulb of thermometer is completely under the liquid being measured and that the line on thermometer is no longer moving before reading To take reading, make sure that your eye is at the level of the liquid on thermometer

Temperature l The SI unit for temperature is Kelvin (abbreviated K) l Zero on the kelvin scale (0 K) is absolute zero, the coldest possible temperature l We can find the temperature in Kelvin by taking our temperature reading in ºC and adding it to 273 25 ºC to K 273 + 25 =298 K

Volume l Volume is the amount of space occupied by an object l The SI base unit is the liter (abbreviated L)

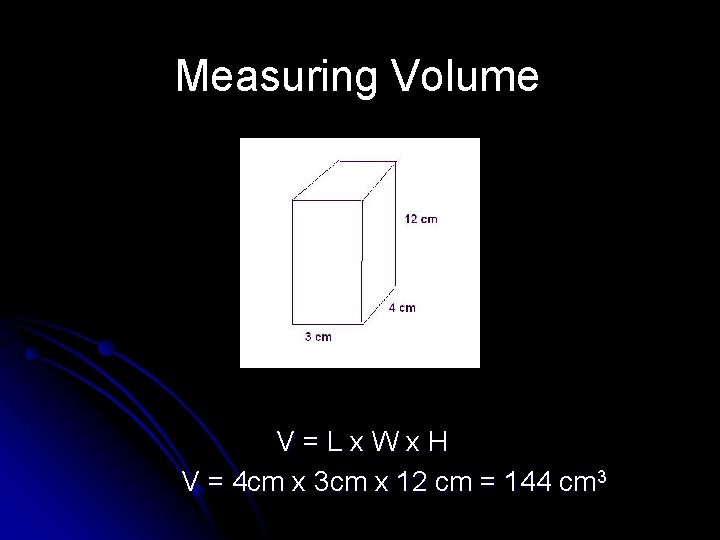
Measuring Volume l Volumes of many solid objects may be calculated by measuring the length, width, and height of the object, then multiplying the numbers together V=Lx. Wx. H

Measuring Volume V=Lx. Wx. H V = 4 cm x 3 cm x 12 cm = 144 cm 3

Measuring Volume l Liquid volumes should be measured with a graduated cylinder (in m. L) l When reading a graduated cylinder, you have to look at the graduated cylinder at eye level l Read the bottom of the meniscus, the curve of the liquid on the cylinder l Read to the nearest unit or half unit

Measuring Volume • Volumes of irregular solid objects may be determined by the displacement method • Put some water in a graduated cylinder and record the volume • Add the object to the graduated cylinder • Record the new volume • The difference between the new volume and the original volume is the volume of the object

Measuring Volume Displacement Method: • http: //www. middleschoolchemistry. com/mul timedia/chapter 3/lesson 2#water_displace ment

Mass • Mass is the amount of matter in an object • The standard unit of mass is the kilogram (abbreviated kg) • Our balances measure mass in grams (g)

Measuring Mass • Measure the mass of a solid using a balance • Balance may be digital or triple beam • Use a weigh “boat” to hold the solid on the pan of the balance

Measuring Mass • Triple beam balance • • • Make sure all of the riders are on 0 Place the boat on the pan Move the rider for the hundreds place one notch at a time until the pointer drops, then move the rider back one notch. Make sure the rider is securely in its notch • Repeat this step with the rider for the tens place and the rider for the ones place

Measuring Mass • Triple beam balance • Now slide the rider for the tenths place until the pointer swings an equal distance on each side of the center mark • Read that rider to the nearest tenth or halfway between (0. 05) • When the pointer matches the center mark, add up the masses shown on the beams

Measuring Mass • Digital balance • • • Make sure the balance reads 0. 0 g If not, press the “Zero”button Measure the mass of the boat and record it Add the solid to the boat Measure the mass of the boat and the solid To find the mass of the solid, subtract the mass of the boat from the mass of the boat and the solid

Measuring Mass • Measure the mass of a liquid using a balance and a graduated cylinder • • • First mass the empty graduated cylinder Add the liquid to the graduated cylinder Mass the liquid and the graduated cylinder together • To get the mass of the liquid, take the mass of the liquid and graduated cylinder and subtract the mass of the graduated cylinder.

Measurement Lab • • • 9 Stations Work in assigned groups Rotate through the stations in order, going to station 1 after you finish station 9 • Be sure to clean up each station and put everything back the way you found it before moving to the next one

Density • Density is the mass per unit volume of a material • Density is calculated using this formula • Density = mass volume • Units are usually g/cm 3