UNIT 1 REVIEW Introduction to biology Scientific methods

UNIT 1 REVIEW • Introduction to biology • • Scientific methods in • Videobiologyhttps: //www. youtube. com/watch? v=yi 0 hw. FDQTSQ • • Types of microscopes • Controlled experiments • Hypotheses and theories • Characteristics of life • Importance of water to life on Earth • Biomolecules found in living organisms

EQ: What is and is not science? Pp 3 +4 *Science is METEOR (acronym for words used) -Measurable, Evidence can be collected, Testable, Empirical (can be tested by experimentation). Observable, Repeatable(tests are repeatable + similar). *Not Science -is negative of METEOR –not testable or any test is not repeatable, no evidence, not measurable. *Also, non scientific includes- opinion, personal taste, imagination, supernatural. EQ: What are the goals of science *understand patterns of nature *explain events of nature * predict events of nature ATTITUDES OF A SCIENTIST- Creativity, Scepticism, Curiousity, Open-mindedness - pp 10 Red Text
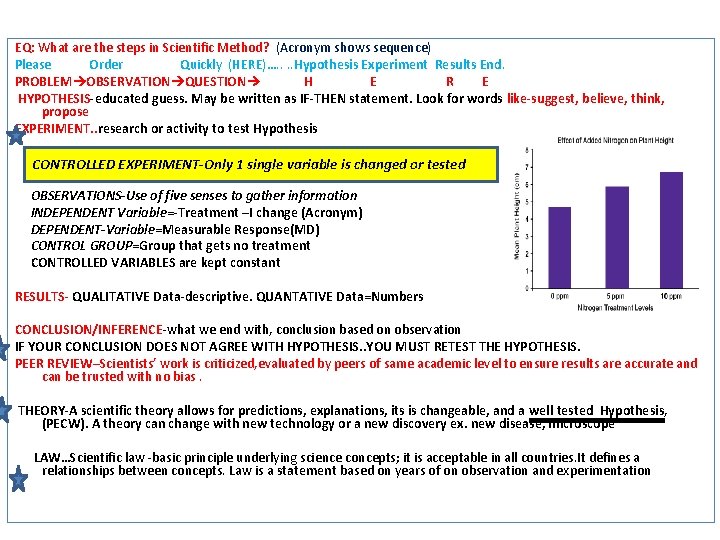
EQ: What are the steps in Scientific Method? (Acronym shows sequence) Please Order Quickly (HERE)…. . Hypothesis Experiment Results End. PROBLEM OBSERVATION QUESTION H E R E HYPOTHESIS-educated guess. May be written as IF-THEN statement. Look for words like-suggest, believe, think, propose EXPERIMENT. . research or activity to test Hypothesis CONTROLLED EXPERIMENT-Only 1 single variable is changed or tested OBSERVATIONS-Use of five senses to gather information INDEPENDENT Variable=-Treatment –I change (Acronym) DEPENDENT-Variable=Measurable Response(MD) CONTROL GROUP=Group that gets no treatment CONTROLLED VARIABLES are kept constant RESULTS- QUALITATIVE Data-descriptive. QUANTATIVE Data=Numbers CONCLUSION/INFERENCE-what we end with, conclusion based on observation IF YOUR CONCLUSION DOES NOT AGREE WITH HYPOTHESIS. . YOU MUST RETEST THE HYPOTHESIS. PEER REVIEW–Scientists’ work is criticized, evaluated by peers of same academic level to ensure results are accurate and can be trusted with no bias. THEORY-A scientific theory allows for predictions, explanations, its is changeable, and a well tested Hypothesis, (PECW). A theory can change with new technology or a new discovery ex. new disease, microscope LAW…Scientific law -basic principle underlying science concepts; it is acceptable in all countries. It defines a relationships between concepts. Law is a statement based on years of on observation and experimentation
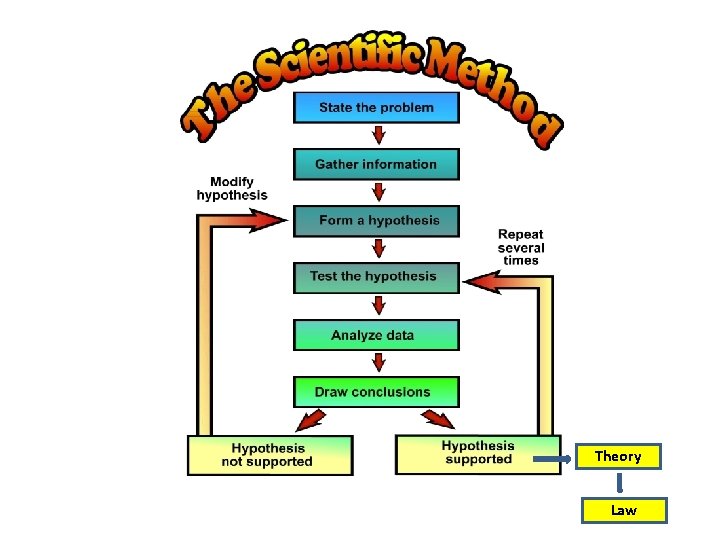
Theory Law

EQ: WHY IS PEER REVIEW IMPORTANT *IN PEER REVIEW–Scientists’ work is scrutinized and evaluated by peers of same academic level to ensure results are of high academic quality and can be trusted and with no bias. *Peer reviewed journals + articles are more credible, trustworthy , more valid , more accurate and believable and have less bias or prejudice. .
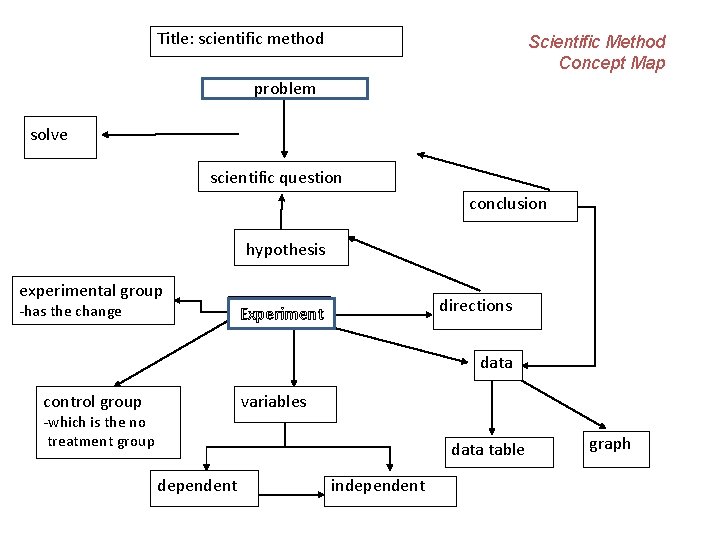
Title: scientific method Scientific Method Concept Map problem solve scientific question conclusion hypothesis experimental group -has the change directions procedure Experiment data control group variables -which is the no treatment group data table dependent independent graph

Science Questions: Pair Activity Read each statement/question below. If it is scientific write a Yes Give a reason for your answer if its not scientific, write a No-Give a reason for your answer Yes-measurable, observable, testable, experiment is repeatable No-Opinion, personal values, imagination, not testable, not measurable, not repeatable #1. Which is heavier, a dog or a rat? #2. Which is prettier a blue flower or a red flower? #3. Which weighs more, a husky or a malamute? #4. How tall is the boogie man? _#5. Which is more comfortable, wool or cotton? #6. How much does Jake weigh? #7. How much does a beluga weigh? #8. How deep is the ocean? #9. Which tastes better plain milk or black coffee? -----#10. What is the circumference of the Earth?
CELL THEORY- EQ: What are the 3 facts of the cell theory-? (Pigs Are Bacon) http: //www. youtube. com/watch? v=dsc. Y_2 QQb. KU 1. All living organisms are made up of cells 2. The cell =basic unit of life 3. All new cells come from preexisting cells Scientists connected to cell discovery *Robert Hooke-discovered cells. Hooke used a light compound microscope to examine the cells *Leeuwenhoek-Observed living cells with better microscopes *Schleiden-notes that plants have cells *Schwan-All living things are made up of cells *Virchow-proposed that all cells came from other cells These scientists put the cell theory together
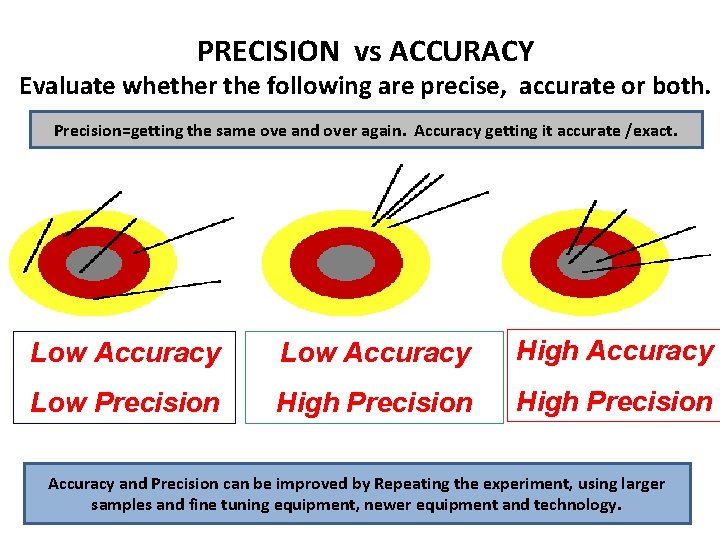
PRECISION vs ACCURACY Evaluate whether the following are precise, accurate or both. Precision=getting the same ove and over again. Accuracy getting it accurate /exact. Low Accuracy High Accuracy Low Precision High Precision Accuracy and Precision can be improved by Repeating the experiment, using larger samples and fine tuning equipment, newer equipment and technology.
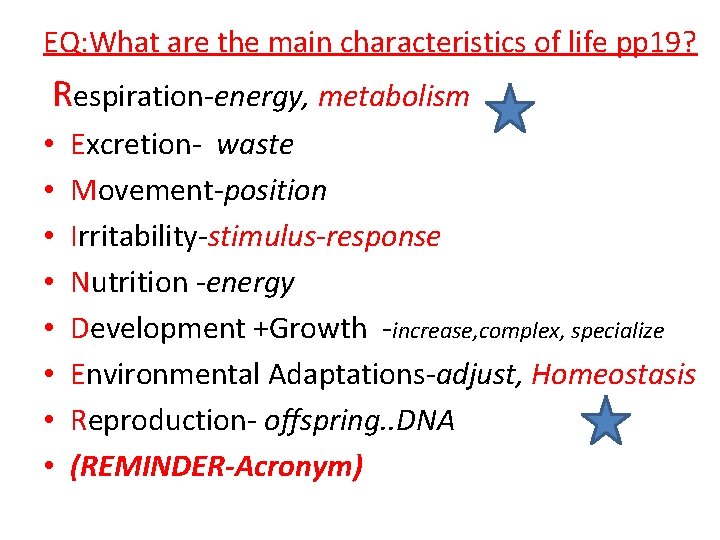
EQ: What are the main characteristics of life pp 19? Respiration-energy, metabolism • • Excretion- waste Movement-position Irritability-stimulus-response Nutrition -energy Development +Growth -increase, complex, specialize Environmental Adaptations-adjust, Homeostasis Reproduction- offspring. . DNA (REMINDER-Acronym)

Vocabulary Word Pairs UNIT 1 1. Scientific + non-scientific /pseudo science 2. Dependent + independent + control (variable) 3 Hypothesis + Theory +Law 4. Observation +data 5. quantitative + qualitative 6. conclusion +inference 7. Support + refute 8. Experimental + control group 9. Controlled Experiment= 10. Theory=pecw 11. Objective+subjective 12. Spontaneous generation +biogenesis
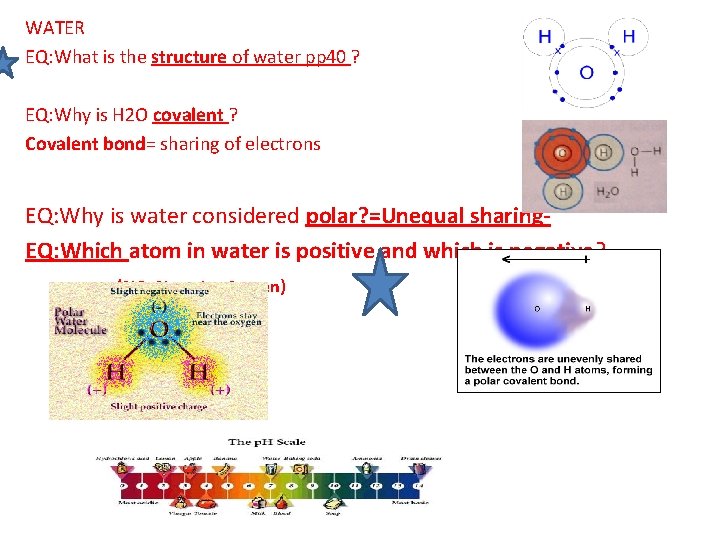
WATER EQ: What is the structure of water pp 40 ? EQ: Why is H 2 O covalent ? Covalent bond= sharing of electrons EQ: Why is water considered polar? =Unequal sharing. EQ: Which atom in water is positive and which is negative? (NO=Negative 0 xygen)

EQ: What are the main properties H 2 O & how do they apply in to living organisms? 1. H Bonding 1. COHESION H 2 O molecules stick to other H 2 O molecules columns, surface tension EQ: Why is H 2 O covalent ? Covalent bond= sharing of electrons EQ: Why is water considered polar? =Unequal sharing. EQ: Which atom in water is positive and which is negative? NO-Neg. O 2 r has a High c Heat) EAT CAPACITY - stasis 2. ADHESION H 2 O molecules stick to other types of molecules. Causes meniscus 3. COHESION +ADHESION Capillary action in plants 4. DENSITY -frozen H 2 Ois less heavy/ dense than liquid H 2 O ice floats 6. SOLVENT-Water dissolves many solutes making solution carry materials thru plants + animals
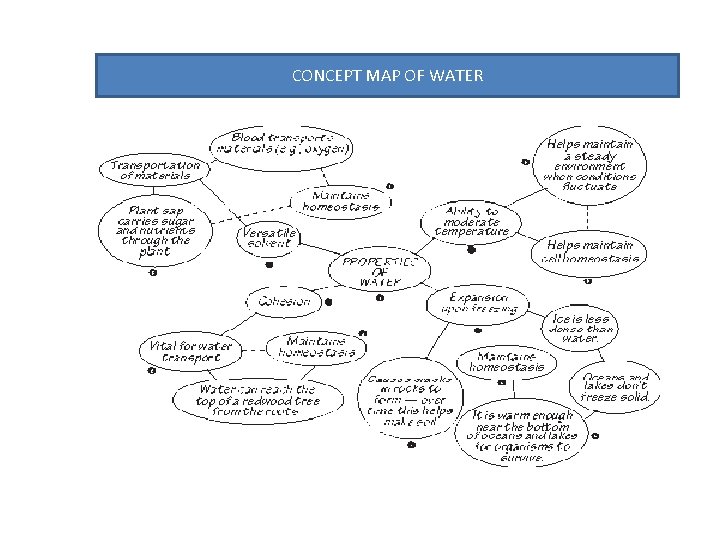
CONCEPT MAP OF WATER
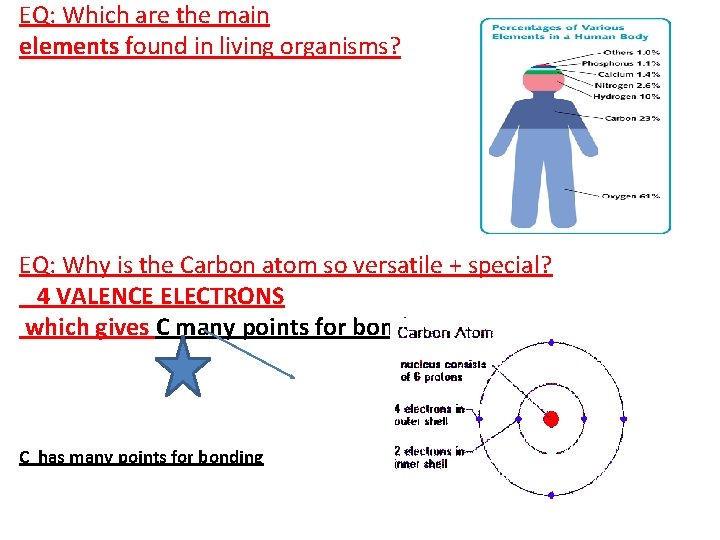
EQ: Which are the main elements found in living organisms? EQ: Why is the Carbon atom so versatile + special? 4 VALENCE ELECTRONS which gives C many points for bonding C has many points for bonding
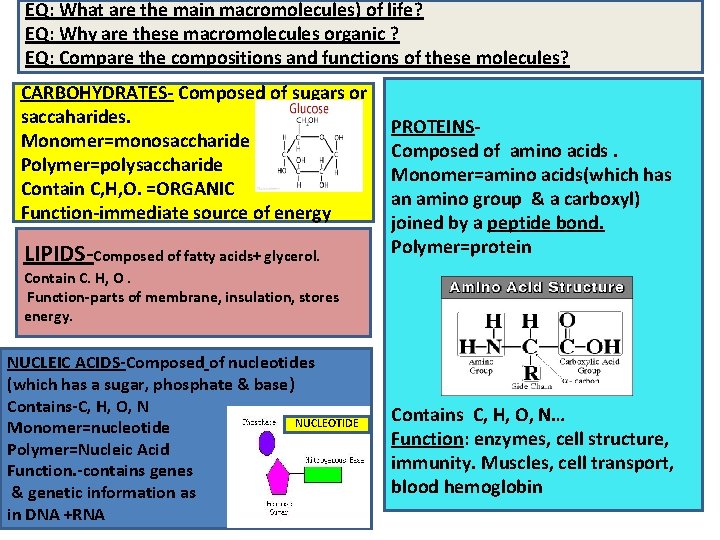
EQ: What are the main macromolecules) of life? EQ: Why are these macromolecules organic ? EQ: Compare the compositions and functions of these molecules? CARBOHYDRATES- Composed of sugars or saccaharides. Monomer=monosaccharide Polymer=polysaccharide Contain C, H, O. =ORGANIC Function-immediate source of energy LIPIDS-Composed of fatty acids+ glycerol. PROTEINSComposed of amino acids. Monomer=amino acids(which has an amino group & a carboxyl) joined by a peptide bond. Polymer=protein Contain C. H, O. Function-parts of membrane, insulation, stores energy. NUCLEIC ACIDS-Composed of nucleotides (which has a sugar, phosphate & base) Contains-C, H, O, N NUCLEOTIDE Monomer=nucleotide E Polymer=Nucleic Acid Function. -contains genes & genetic information as in DNA +RNA Contains C, H, O, N… Function: enzymes, cell structure, immunity. Muscles, cell transport, blood hemoglobin
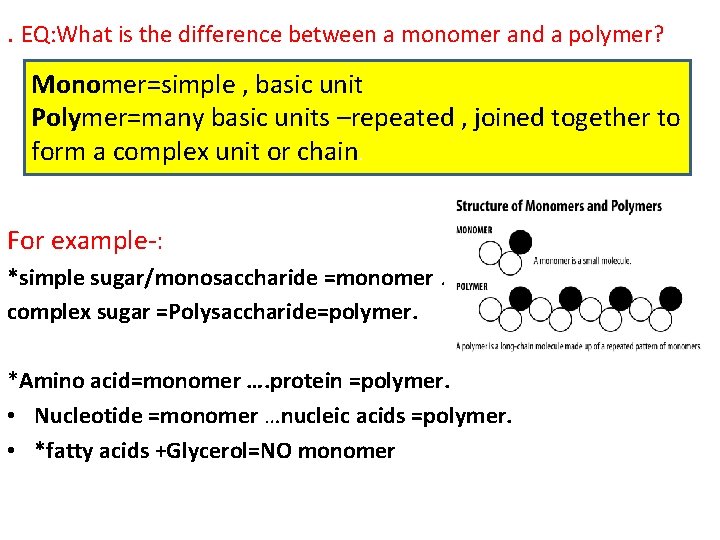
. EQ: What is the difference between a monomer and a polymer? Monomer=simple , basic unit Polymer=many basic units –repeated , joined together to form a complex unit or chain. For example-: *simple sugar/monosaccharide =monomer. complex sugar =Polysaccharide=polymer. *Amino acid=monomer …. protein =polymer. • Nucleotide =monomer …nucleic acids =polymer. • *fatty acids +Glycerol=NO monomer
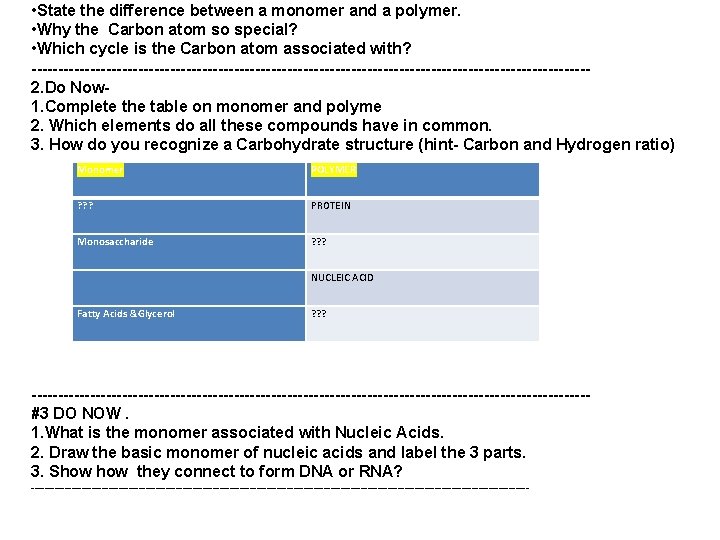
• State the difference between a monomer and a polymer. • Why the Carbon atom so special? • Which cycle is the Carbon atom associated with? ----------------------------------------------------2. Do Now 1. Complete the table on monomer and polyme 2. Which elements do all these compounds have in common. 3. How do you recognize a Carbohydrate structure (hint- Carbon and Hydrogen ratio) Monomer POLYMER ? ? ? PROTEIN Monosaccharide ? ? ? NUCLEIC ACID Fatty Acids &Glycerol ? ? ? ----------------------------------------------------#3 DO NOW. 1. What is the monomer associated with Nucleic Acids. 2. Draw the basic monomer of nucleic acids and label the 3 parts. 3. Show they connect to form DNA or RNA? --------------------------------------------------------------------------
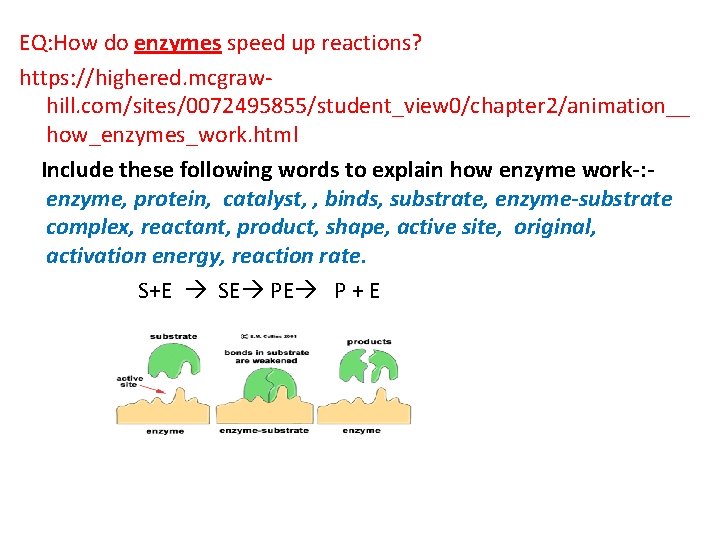
EQ: How do enzymes speed up reactions? https: //highered. mcgrawhill. com/sites/0072495855/student_view 0/chapter 2/animation__ how_enzymes_work. html Include these following words to explain how enzyme work-: enzyme, protein, catalyst, , binds, substrate, enzyme-substrate complex, reactant, product, shape, active site, original, activation energy, reaction rate. S+E SE PE P + E
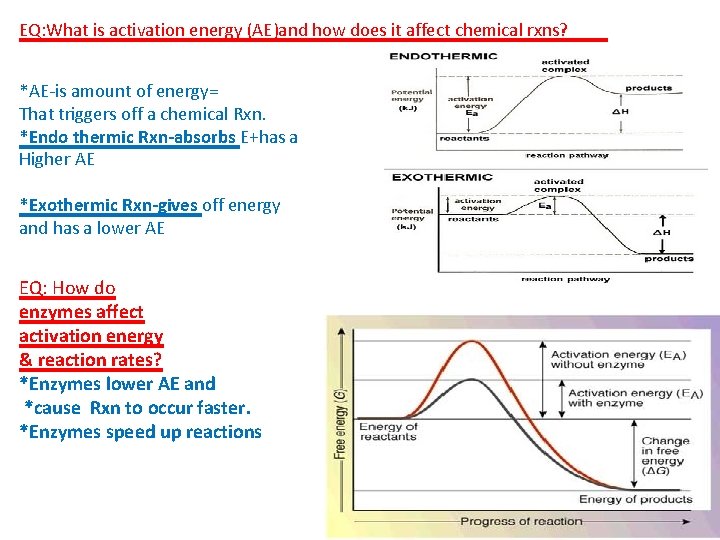
EQ: What is activation energy (AE)and how does it affect chemical rxns? *AE-is amount of energy= That triggers off a chemical Rxn. *Endo thermic Rxn-absorbs E+has a Higher AE *Exothermic Rxn-gives off energy and has a lower AE EQ: How do enzymes affect activation energy & reaction rates? *Enzymes lower AE and *cause Rxn to occur faster. *Enzymes speed up reactions
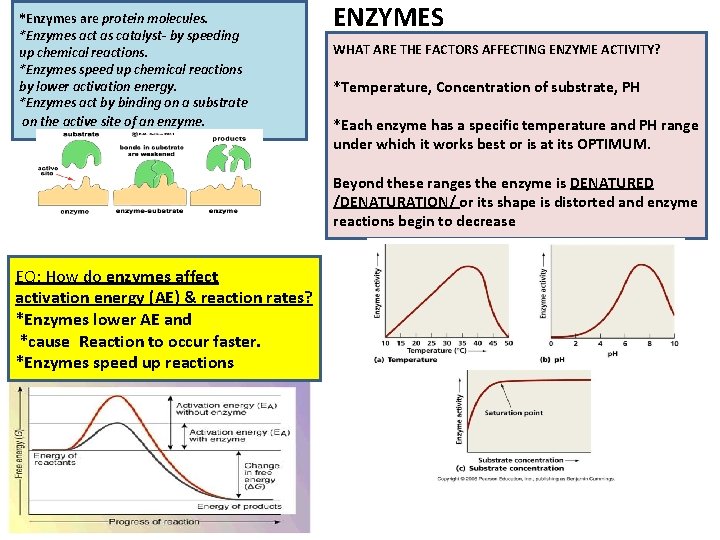
*Enzymes are protein molecules. *Enzymes act as catalyst- by speeding up chemical reactions. *Enzymes speed up chemical reactions by lower activation energy. *Enzymes act by binding on a substrate on the active site of an enzyme. ENZYMES WHAT ARE THE FACTORS AFFECTING ENZYME ACTIVITY? *Temperature, Concentration of substrate, PH *Each enzyme has a specific temperature and PH range under which it works best or is at its OPTIMUM. Beyond these ranges the enzyme is DENATURED /DENATURATION/ or its shape is distorted and enzyme reactions begin to decrease EQ: How do enzymes affect activation energy (AE) & reaction rates? *Enzymes lower AE and *cause Reaction to occur faster. *Enzymes speed up reactions
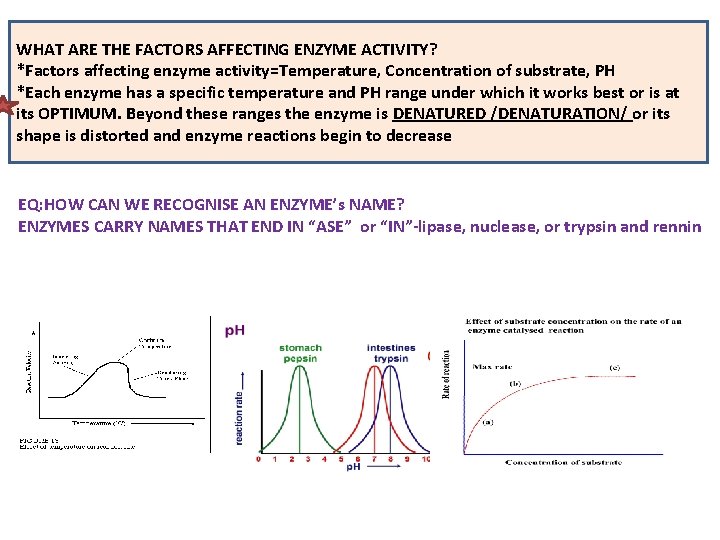
WHAT ARE THE FACTORS AFFECTING ENZYME ACTIVITY? *Factors affecting enzyme activity=Temperature, Concentration of substrate, PH *Each enzyme has a specific temperature and PH range under which it works best or is at its OPTIMUM. Beyond these ranges the enzyme is DENATURED /DENATURATION/ or its shape is distorted and enzyme reactions begin to decrease EQ: HOW CAN WE RECOGNISE AN ENZYME’s NAME? ENZYMES CARRY NAMES THAT END IN “ASE” or “IN”-lipase, nuclease, or trypsin and rennin
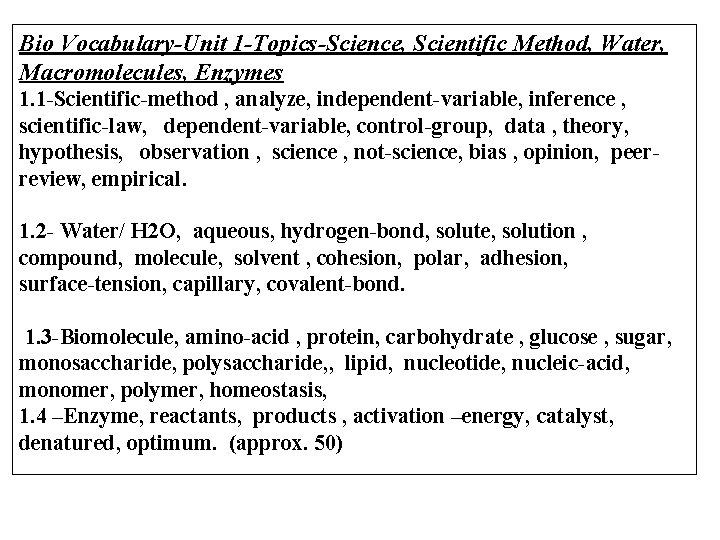
Bio Vocabulary-Unit 1 -Topics-Science, Scientific Method, Water, Macromolecules, Enzymes 1. 1 -Scientific-method , analyze, independent-variable, inference , scientific-law, dependent-variable, control-group, data , theory, hypothesis, observation , science , not-science, bias , opinion, peerreview, empirical. 1. 2 - Water/ H 2 O, aqueous, hydrogen-bond, solute, solution , compound, molecule, solvent , cohesion, polar, adhesion, surface-tension, capillary, covalent-bond. 1. 3 -Biomolecule, amino-acid , protein, carbohydrate , glucose , sugar, monosaccharide, polysaccharide, , lipid, nucleotide, nucleic-acid, monomer, polymer, homeostasis, 1. 4 –Enzyme, reactants, products , activation –energy, catalyst, denatured, optimum. (approx. 50)

IMPORTANT PREFIXES *AUTO-self (autotroph) *AQUA-water (aquatic) * BIO- life (biotic, biosphere) • CO-together, share, 2, (covalent, codominant, correlation, • HYDRO-water • EX-out of (exothermic) and EN-in (endothermic) • INTER=between, among, connected (interrelated, interconnected) • MACRO =large…MICRO =little (macromolecule, microscope) • MONO=one (monomer)…POLY=many(polymer)…. DI-two • HOMO=same(homozygous)…. HETERO=different(heterozygous) • HOMEO-same (homeostatis) • PHOTO=light (photosynthesis) • CHEMO-chemical • A, AN, IN, NON-opposite-abiotic, independent, anaerobic

Learning Objectives -Students will be able to… 1 -1: Distinguish between what is science and what is not. (SC. 912. N. 2. 1) 1 -2: Differentiate between the types of questions science can and cannot address. (SC. 912. N. 2. 2) 1 -3: Formulate testable questions and hypotheses. (SC. 912. N. 1. 1, SC. 912. N. 2. 2) 1 -4: Evaluate the reliability of sources of scientific information. (SC. 912. N. 1. 4) 1 -5: Explain the role of controlled experiments in a scientific explanation. (SC. 912. N. 1. 1) 1 -6: Design a controlled experiment to solve a problem or investigate a question. (SC. 912. N. 1. 1) 1 -7: Make systematic observations and record data. (SC. 912. N. 1. 1) 1 -8: Demonstrate the proper use and care of a microscope. (SC. 912. N. 1. 1) 1 -9: Compare types of scientific investigations. (SC. 912. N. 1. 1) 1 -10: Explain why a water molecule is polar and able to form hydrogen bonds. (SC. 912. L. 18. 12) 1 -11: Describe how hydrogen bonding affects the behavior of water molecules. (SC. 912. L. 18. 12) 1 -12: Evaluate the biological significance of the cohesiveness of water. (SC. 912. L. 18. 12) 1 -13: Describe the role of water as a solvent in organisms and the environment. (SC. 912. L. 18. 12) 1 -14: Analyze how the expansion of water upon freezing affects living systems. (SC. 912. L. 18. 12) 1 -15: Evaluate the biological significance of water’s ability to moderate its temperature. (SC. 912. L. 18. 12) 1 -16: Organize, represent, and analyze data. (SC. 912. N. 1. 1) 1 -17: Formulate and justify conclusions using evidence from data. (SC. 912. N. 1. 1, SC. 912. N. 1. 6) 1 -18: List the major elements found in living things. (SC. 912. L. 18. 1) 1 -19: Relate the properties of carbon to the structure of its atom. (SC. 912. L. 18. 1) 1 -20: Name the four classes of organic molecules found in living things. (SC. 912. L. 18. 1) 1 -21: Evaluate experimental design, identify sources of error, and suggest improvements. (SC. 912. N. 1. 1) 1 -22: Explain the importance of the communication of scientists’ procedures and results. (SC. 912. N. 1. 3) 1 -23: Describe the process of validating scientists’ work. (SC. 912. N. 1. 3) 1 -24: Describe how a scientific theory develops. (SC. 912. N. 3. 1, SC. 912. N. 3. 2) 1 -25: Describe the basic molecular structure of proteins. (SC. 912. L. 18. 1) 1 -26: Describe how an enzyme’s shape is important to its function. (SC. 912. L. 18. 1, SC. 912. L. 18. 11) 1 -27: Identify the relationship between activation energy and chemical reactions. (SC. 912. L. 18. 11) 1 -28: Explain the role of enzymes as catalysts in biochemical reactions. (SC. 912. L. 18. 11) 1 -29: Investigate and identify the effects of various factors (e. g. , p. H, temperature) on enzyme activity. (SC. 912. L. 18. 11, SC. 912. N. 1. 1)

OBJECTIVES /STANDARDS (enlarged) SCIENCE +SCIENTIFIC METHOD * 1 -1: Distinguish between what is science and what is not. (SC. 912. N. 2. 1) • 1 -2: Differentiate between the types of questions science can and cannot address. (SC. 912. N. 2. 2) • 1 -3: Formulate testable questions and hypotheses. (SC. 912. N. 1. 1, SC. 912. N. 2. 2) • 1 -4: Evaluate the reliability of sources of scientific information. (SC. 912. N. 1. 4) • 1 -5: Explain the role of controlled experiments in a scientific explanation. (SC. 912. N. 1. 1) • 1 -6: Design a controlled experiment to solve a problem or investigate a question. (SC. 912. N. 1. 1) • 1 -7: Make systematic observations and record data. (SC. 912. N. 1. 1) • 1 -8: Demonstrate the proper use and care of a microscope. (SC. 912. N. 1. 1) • 1 -9: Compare types of scientific investigations. (SC. 912. N. 1. 1) • 1 -16: Organize, represent, and analyze data. (SC. 912. N. 1. 1) • 1 -17: Formulate and justify conclusions using evidence from data. (SC. 912. N. 1. 1, SC. 912. N. 1. 6 • 1 -21: Evaluate experimental design, identify sources of error, and suggest improvements. (SC. 912. N. 1. 1) • 1 -22: Explain the importance of the communication of scientists’ procedures and results. (SC. 912. N. 1. 3) • 1 -23: Describe the process of validating scientists’ work. (SC. 912. N. 1. 3) • 1 -24: Describe how a scientific theory develops. (SC. 912. N. 3. 1, SC. 912. N. 3. 2)

WATER +WATER PROPERTIES 1 -10: Explain why a water molecule is polar and able to form hydrogen bonds. (SC. 912. L. 18. 12) 1 -11: Describe how hydrogen bonding affects the behavior of water molecules. (SC. 912. L. 18. 12) 1 -12: Evaluate the biological significance of the cohesiveness of water. (SC. 912. L. 18. 12) 1 -13: Describe the role of water as a solvent in organisms and the environment. (SC. 912. L. 18. 12) 1 -14: Analyze how the expansion of water upon freezing affects living systems. (SC. 912. L. 18. 12) 1 -15: Evaluate the biological significance of water’s ability to moderate its temperature. (SC. 912. L. 18. 12)
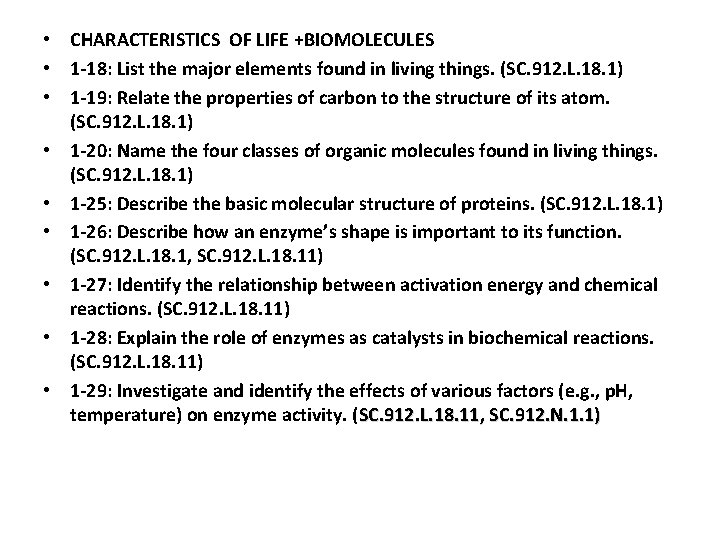
• CHARACTERISTICS OF LIFE +BIOMOLECULES • 1 -18: List the major elements found in living things. (SC. 912. L. 18. 1) • 1 -19: Relate the properties of carbon to the structure of its atom. (SC. 912. L. 18. 1) • 1 -20: Name the four classes of organic molecules found in living things. (SC. 912. L. 18. 1) • 1 -25: Describe the basic molecular structure of proteins. (SC. 912. L. 18. 1) • 1 -26: Describe how an enzyme’s shape is important to its function. (SC. 912. L. 18. 1, SC. 912. L. 18. 11) • 1 -27: Identify the relationship between activation energy and chemical reactions. (SC. 912. L. 18. 11) • 1 -28: Explain the role of enzymes as catalysts in biochemical reactions. (SC. 912. L. 18. 11) • 1 -29: Investigate and identify the effects of various factors (e. g. , p. H, temperature) on enzyme activity. (SC. 912. L. 18. 11, SC. 912. N. 1. 1)


EOC PRACTICE UNIT 1 pp 2

UNIT 1 QUESTIONSBlack- Science + Sc. Mths. Red=Biomolecules Blue=Water 1. Information gathered from observing a plant grow 3 cm over a two-week period is called Slide 3 Inferences B. variables C. Hypotheses D. data 2. The work of scientists usually begins with Slide 3 A. testing a hypothesis B. careful observations C. creating experiments D. drawing conclusions 3. A hypothesis is Slide 3 A. a prediction that something is uncertain B. a testable proposition that explains a phenemon or answers a question C. a proven scientific fact D. a design of an experiment that can be used for the process of science 4. The process by which several scientists review another researcher’s work prior to publication to quality and accuracy is called Slide 5 A. Hypothesis B. data C. peer review D. critical analysis 5. A controlled experiment allows a scientist to test. Slide 3 A. a conclusion B. a mass of information C. several variables D. one variable 6. Which of the following is NOT a goal of science? Slide 2 A. to investigate and understand the natural world B. to explain events in the natural world C. to establish a collection of unchanging truths D. to use derived explanations to make useful predictions 7. During a controlled experiment, a scientist isolates and tests Slide 3 A. conclusion B. mass of information C. control group 8. A scientific hypothesis- Slide 3 A. can be based on personal beliefs or opinions C. does not have to be tested to be accepted as correct D. a single variable B. can be tested by experiments or observations D. is a proven fact with much evidence to support it 9. You suggest that the presence of water could speed up the growth of mold on bread. This is a (an) Slide 3 A. Conclusion B. Hypothesis C. experiment D. analysis 10. Suppose that a scientist proposes a hypothesis about how a newly discovered virus affects humans. Other virus researchers would likely Slide 3 A. reject the hypothesis right away. B. change the hypothesis to fit their own findings. C. design new experiments to test the proposed hypothesis. D. assume that the hypothesis is true for all viruses.

11. Which of the following steps to solve a problem must be completed first? Slide 3 +4 A. analyzing data B. recognizing + identifying the problem C. forming a hypothesis D. testing a hypothesis 12. Which skill are you using when you use your five senses to gather information? Slide 3 A. posing questions B. Observing C. developing hypotheses D. designing experiments 13. In a controlled experiment, a scientist is studying how long it takes parachutes of different sizes to fall to the ground. What is the manipulated variable? Slide 3 A. the size of the parachute B. the height from which the parachute is dropped C. the size of the object carried by the parachute D. the time it takes for the parachute to drop 14. Which sentence best describes a scientific theory? Slide 3 A. It never changes. B. It changes every time it is tested. C. It can be proven conclusively. D. It is well-tested+explains a range of observations. 15. Scientific theories can be changed or replaced when Slide 3 A. New technology is invented B. new discovery is made C. Scientists decide to work on different problems D. Scientists make models 16. Peer reviewing a researcher’s scientific findings ensures Slide 5 A. That the work will be funded B. that the findings are free from biases, unfair influences, fraud and mistakes C. That the findings are written in proper grammar D. That all the findings will be published. 17. Scientific skeptism means that Slide 2 A. evidence must be based on the logic +mutual agreement of expert scientists B. scientists evaluate ideas on the basis of personal logic, C. scientists are skeptical about what they cannot see themselves D. ideas must be evaluated on the basis of careful logic and investigation 18. Before accepting the claims of a scientist, it is worthwhile to Slide 5 A. evaluate the credentials of the individual C. examine the reputation of the institution represented by the person B. examine the researcher's source of funding D. all of the above 19. When looking for scientific information the best option is to read/ search Slide 5 A. Newspaper articles B. Text books C. Peer-reviewed scientific journals. D. Ask a friend about the topic 20. Which sentence best states the importance of using control groups? Slide 3 A. Control groups provide a method by which statistical variability can be reduced. B. Control groups eliminate the need for statistical tests and simplify calculations. C. Control groups allow comparison between subjects receiving a treatment and those receiving no treatment. D. Control groups eliminate the need for large sample sizes, reducing the number of measurements needed.

UNIT 1 All water questions –Study Slides 12 & 13 21. Water is essential for life. Its properties make H 2 O the single most important molecule in plant life. Which of the following properties of H 2 O enables it to move from the roots to the leaves of plants? Pp 40 -41 A. Water expands as it freezes. B Water is an excellent solvent. C. Water exhibits cohesive behavior. D. Water is able to moderate temperature. 22. Large bodies of water, such as lakes and oceans, do not quickly fluctuate in temperature. Pp 40 -41 What is the reason for this phenomenon? A. Water is an acid. B. Water is a versatile solvent. C. Water has a high heat capacity. D. Water acts as a buffer. 23. Why does ice stay at the top of oceans instead of sinking to the bottom? Pp 40 -41 A. Ice is colder than liquid water. B. Ice is less dense than liquid water. C. Ice is more dense than liquid water. D. Ice is warmer than liquid water. 24. Water is often called the "universal solvent" because many substances can be dissolved in water. pp 42 What property of water allows it to be such a versatile solvent? A. purity B. polarity and cohesion C. high heat capacity D. expansion upon freezing 25. Water is co-valent because the electrons are shared but it is considered polar because: pp 40 A. Electrons are share equally by hydrogen and oxygen atoms B. Electrons are exchanged between hydrogen and oxygen atoms. C. Electrons are unequally shared between hydrogen and oxygen D. The electons form a hydrogen bonds 26. Water molecules are polar, with the pp 41 A. oxygen side being slightly positive and the hydrogen side being slightly negative. B. oxygen and hydrogen sides being slightly positive. C. oxygen and hydrogen sides being slightly negative. D. oxygen side being slightly negative and the hydrogen side being slightly positive. 27. Which characteristic of H 2 O allows insects, like the water strider, to walk on the surface of water? pp 41 A. PH B. solubility C. Cohesion D. Heat capacity

UNIT 1 pp 5 28. Some adult insects are unable to swim but are able to walk on top of water. What characteristic of water enables these insects to walk on top of water? Pp 40 -41 Slide 13 A. p. H B. solvent properties C. atomic bonds D. surface tension 29. Which term refers to water’s attraction to other substances that have full or partial electrical charges? Slide 13 A. Polarity B. Adhesion C. Cohesion D. Solvent 30. A general definition for “cohesion” is the attraction of: pp 40 -41 Slide 13 A. particles of the same substance B. particles of neither the same nor different substances C. particles of both the same and different substances D. particles of different substances 31. The p. H of distilled water is: pp 43 A. 0 B. 1 C. 5 D. 7 32. Which property of water allows it to travel in a column against the force of gravity in tall trees ? p 40 -41 A. Polarity B. H+ bonding C. Cohesion D. Solvent 33. Which property of water allows a curved surface or meniscus to be formed in a graduated cylinder? P 40 -41 A. Polarity B. Adhesion C. Cohesion D. Solvent 34. Which of the following statements about water is false? Pp 40 -41 A. water molecules are polar B. it takes very little heat to change the temperature of water C. all living organisms contain water D. none; all these statements are true

EOC PRACTICE UNIT 1 pp 3 35. The molecules associated with energy in the cell are mainly -: Slide 16 A. carbohydrates + proteins B. fats and carbohydrates C. proteins and nucleic acids D. carbohydrates + nucleic acids 36. RNA and DNA are which type of organic compound? Pp 48 A. Carbohydrate B. lipid C. nucleic acid Slide 16 D. protein 37. A sugar, a phosphate group, and a nitrogen base form the building blocks of which organic compound? Pp 48 Slide 16 A. Carbohydrates B. lipids C. Nucleic acids D. Proteins 38. Why do most enzymes not function properly after being exposed to high temperatures? pp 50 -52. Slide 19 -21 A. They have been converted to tripeptides. B. Their water content has been reduced. C Their bonding structure has been changed. D. They have combined with another enzyme 39. The basic monomer in nucleic acids are. Slide 16 +17 A. Sugars 40. Nucleotides are composed of-: Slide 16 A. Base +amino acid +nitrogen B. Base+sugar +phosphate B. nucleotides C. amino-acids D. fatty acids C. sugar +fatty acid +phosphate 41. Why is carbon so special compared to other elements? Pp 45 Slide 15 A. carbon atoms can bond to one another and form a lot of different structures B. carbon atoms have four valence electrons and can form quadruple bonds C. only carbon atoms can form covalent bonds with oxygen and hydrogen D. only carbon atoms can be dissolved in water solutions and suspensions 42. What are building blocks of proteins? Pp 48 A. amino acids B. simple sugar Slide 16 C. fatty acids D. Nucleotides 43. What will most likely happen if an appropriate enzyme is added to a chemical reaction? Pp 50 -52 Slide 19 -21 A. The reaction rate will increase. B. The equilibrium of the reaction will be maintained. C. The reaction rate will decrease. D. The reaction will stop 44. Which factors below can denature an enzyme and destroy its ability to speed up reactions. Slide 19 -21 A. Temperature and PH B. PH and glucose C. pressure and space D. none of the above 45. What is the relationship between enzymes and activation energy? Slide 19 -21 A. Enzymes increase activation energy B. Enzymes lower activation energy. C. Enzymes do not affect activation energy

EOC PRACTICE UNIT 1 pp 6
- Slides: 36