Unit 1 Polymer Chemistry CH 1121 The Bonding

Unit 1: Polymer Chemistry CH 1121
The Bonding of Carbon � To the right of the staircase on the periodic table ◦ It is a non-metal � Has 4 valence electrons meaning it will form 4 bonds � Carbon forms a variety of covalent compounds with other carbon atoms or other non-metals
The Bonding of Carbon � As was covered in CH 1120, carbon can form polar covalent bonds � In polar covalent bonds carbon and the other atom in the bond have an electronegativity difference (ΔEN) between 0. 4 and 2. 0
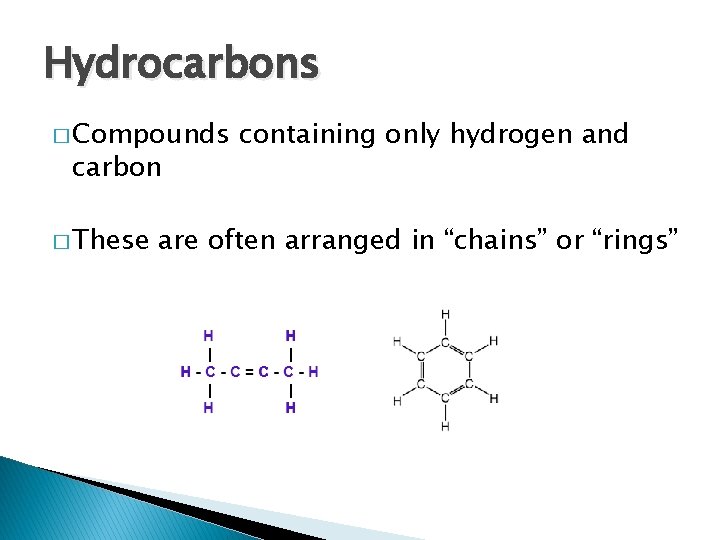
Hydrocarbons � Compounds carbon � These containing only hydrogen and are often arranged in “chains” or “rings”
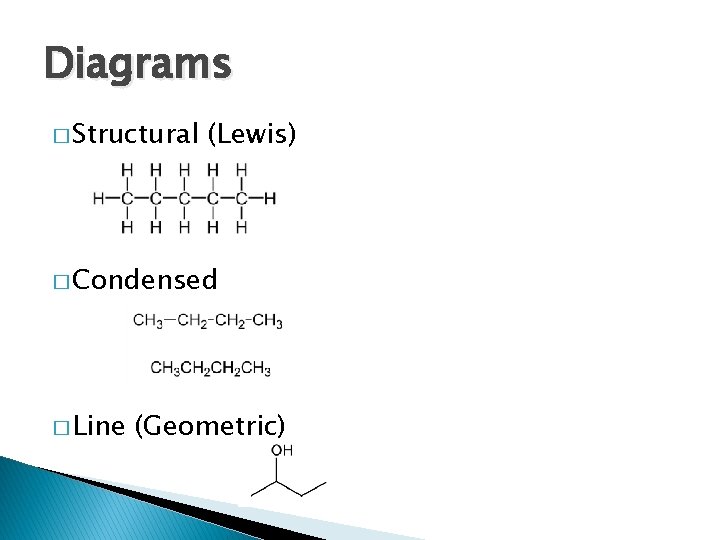
Diagrams � Structural (Lewis) � Condensed � Line (Geometric)
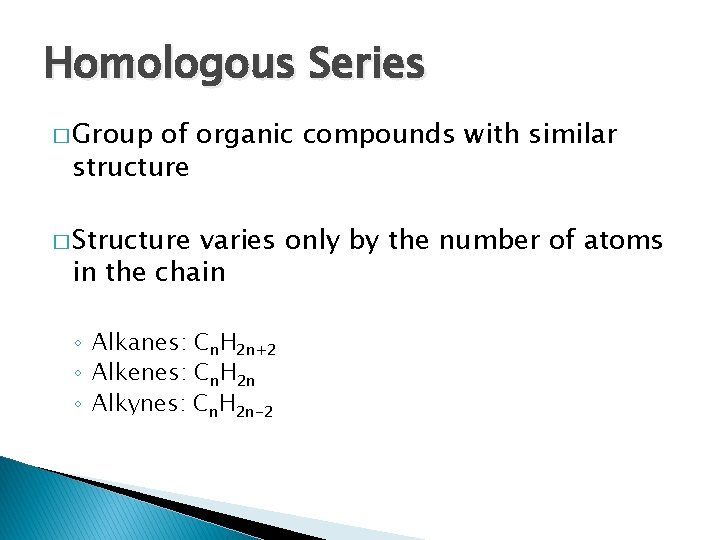
Homologous Series � Group of organic compounds with similar structure � Structure varies only by the number of atoms in the chain ◦ Alkanes: Cn. H 2 n+2 ◦ Alkenes: Cn. H 2 n ◦ Alkynes: Cn. H 2 n-2
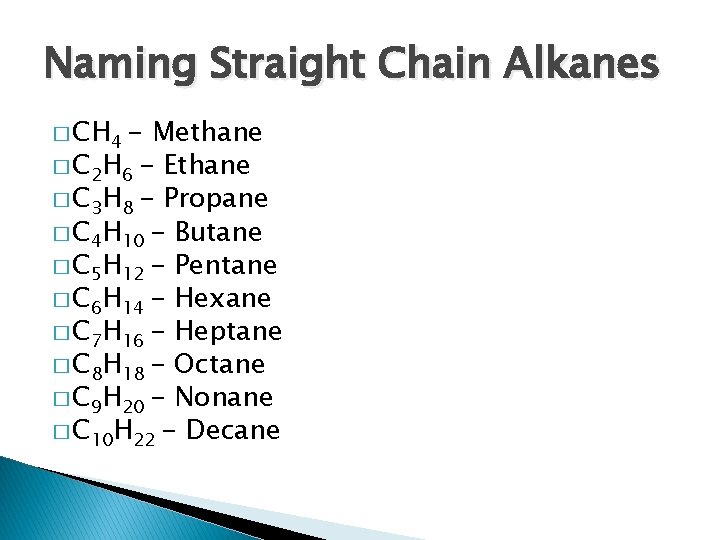
Naming Straight Chain Alkanes � CH 4 - Methane � C 2 H 6 - Ethane � C 3 H 8 - Propane � C 4 H 10 - Butane � C 5 H 12 - Pentane � C 6 H 14 - Hexane � C 7 H 16 - Heptane � C 8 H 18 - Octane � C 9 H 20 - Nonane � C 10 H 22 - Decane
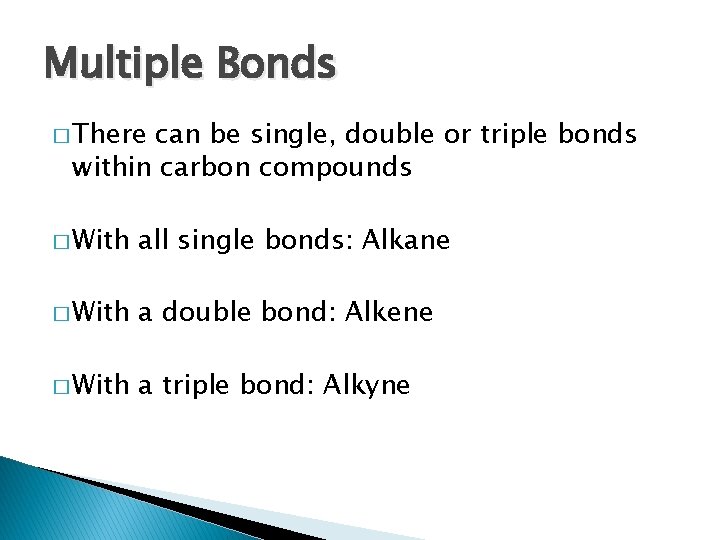
Multiple Bonds � There can be single, double or triple bonds within carbon compounds � With all single bonds: Alkane � With a double bond: Alkene � With a triple bond: Alkyne
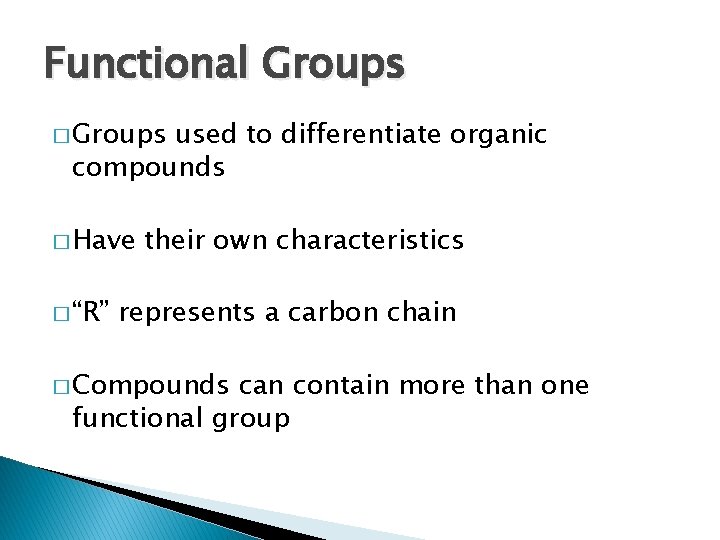
Functional Groups � Groups used to differentiate organic compounds � Have � “R” their own characteristics represents a carbon chain � Compounds can contain more than one functional group
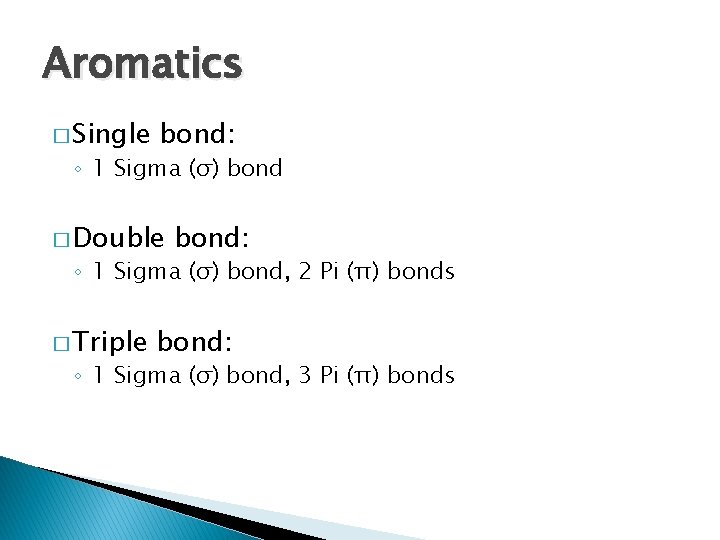
Aromatics � Single bond: ◦ 1 Sigma (σ) bond � Double bond: ◦ 1 Sigma (σ) bond, 2 Pi (π) bonds � Triple bond: ◦ 1 Sigma (σ) bond, 3 Pi (π) bonds
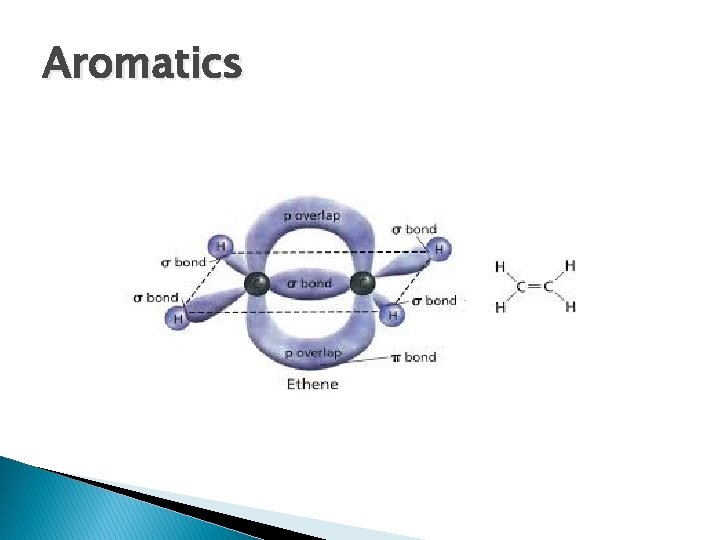
Aromatics
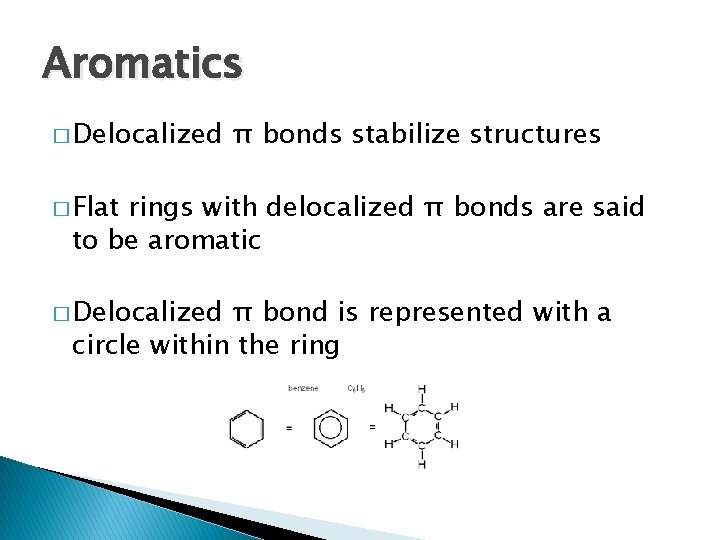
Aromatics � Delocalized π bonds stabilize structures � Flat rings with delocalized π bonds are said to be aromatic � Delocalized π bond is represented with a circle within the ring

Aromatics
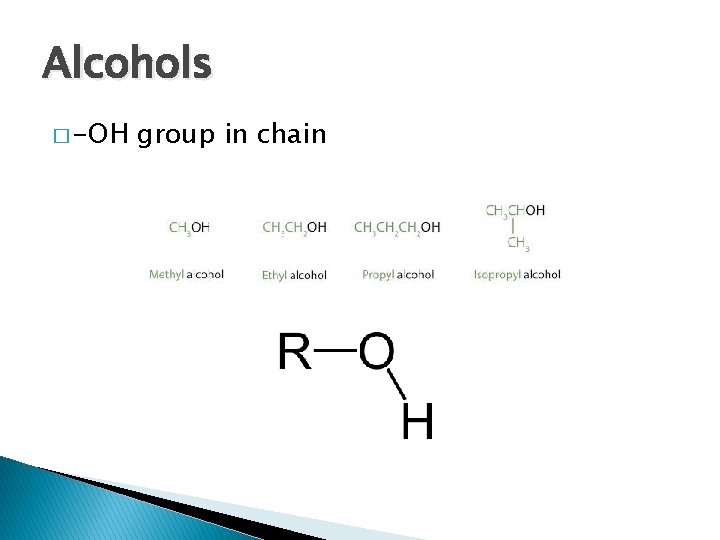
Alcohols � -OH group in chain
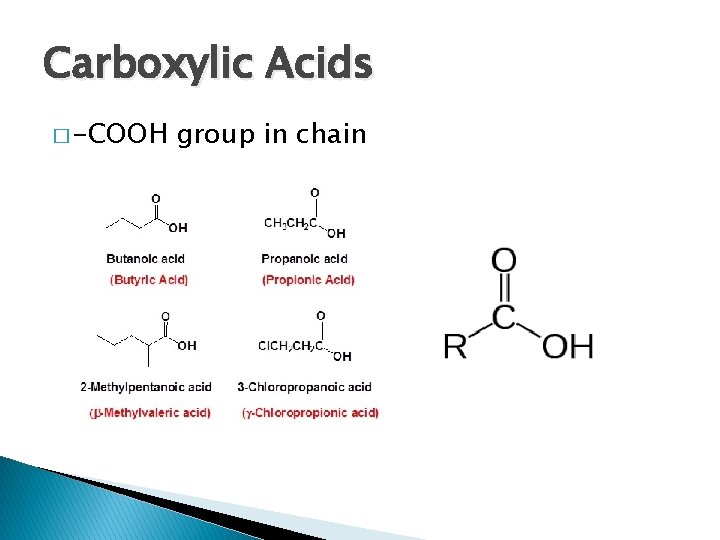
Carboxylic Acids � -COOH group in chain
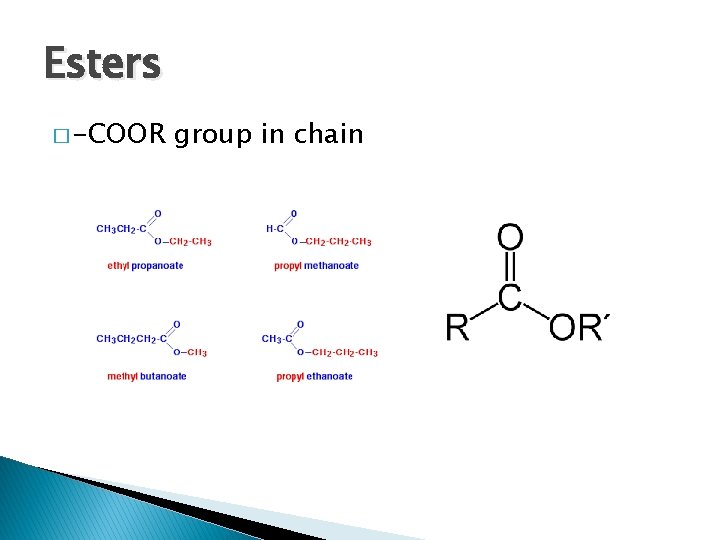
Esters � -COOR group in chain
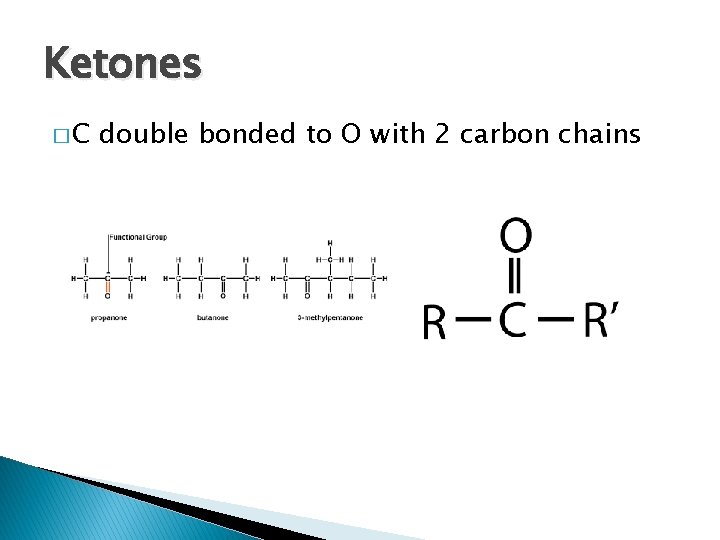
Ketones �C double bonded to O with 2 carbon chains
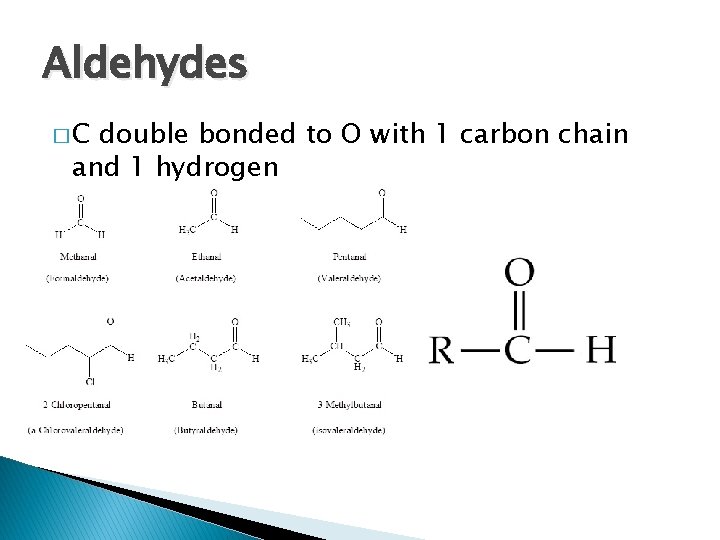
Aldehydes �C double bonded to O with 1 carbon chain and 1 hydrogen
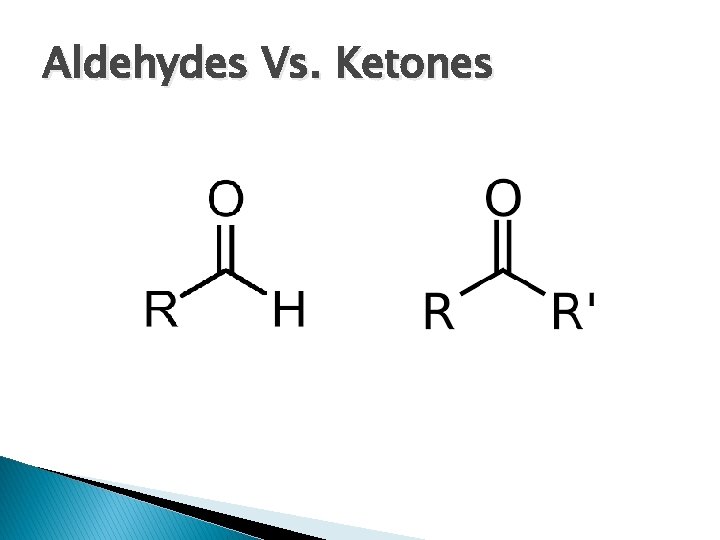
Aldehydes Vs. Ketones
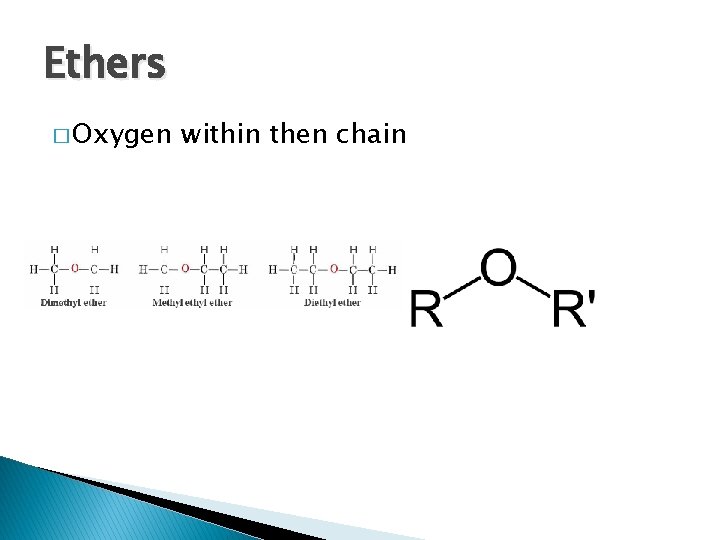
Ethers � Oxygen within then chain
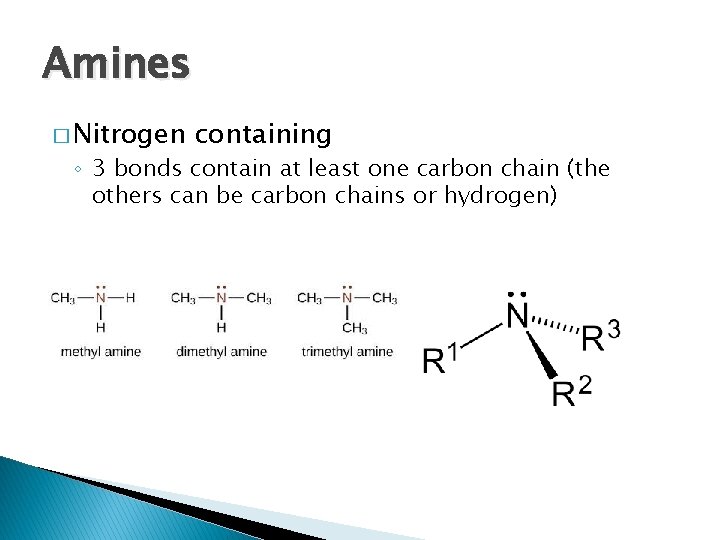
Amines � Nitrogen containing ◦ 3 bonds contain at least one carbon chain (the others can be carbon chains or hydrogen)
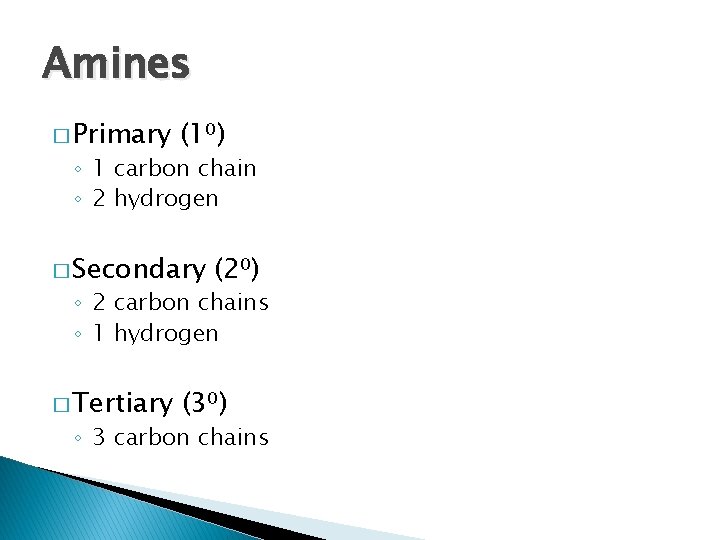
Amines � Primary (1⁰) ◦ 1 carbon chain ◦ 2 hydrogen � Secondary (2⁰) ◦ 2 carbon chains ◦ 1 hydrogen � Tertiary (3⁰) ◦ 3 carbon chains
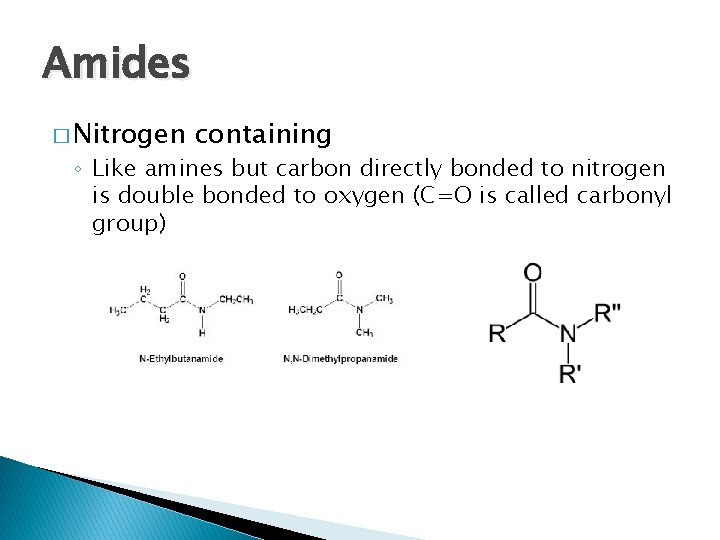
Amides � Nitrogen containing ◦ Like amines but carbon directly bonded to nitrogen is double bonded to oxygen (C=O is called carbonyl group)
Polymers � AKA Plastics � Large molecules (macromolecules) made of many repeating units � Formed through polymerization � Natural ◦ Wool, silk, biopolymers (carbohydrates, proteins) � Synthetic (focus of this course)
Monomers � “one part” � Molecule that chemically bonds to other molecules to create a polymer
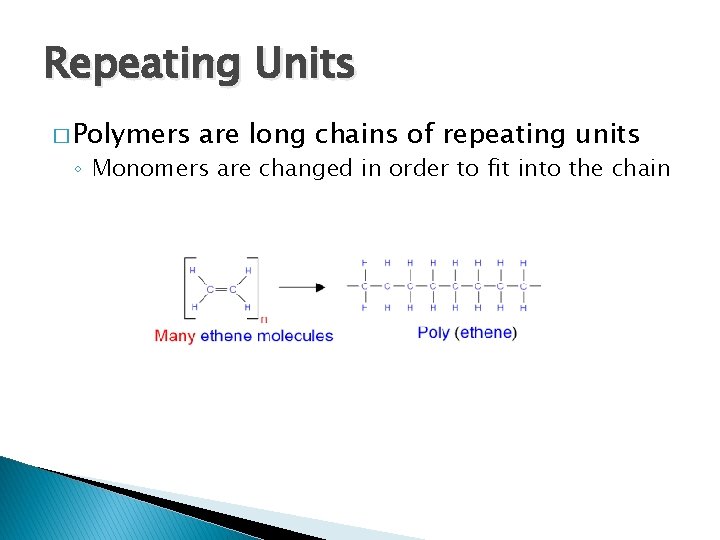
Repeating Units � Polymers are long chains of repeating units ◦ Monomers are changed in order to fit into the chain
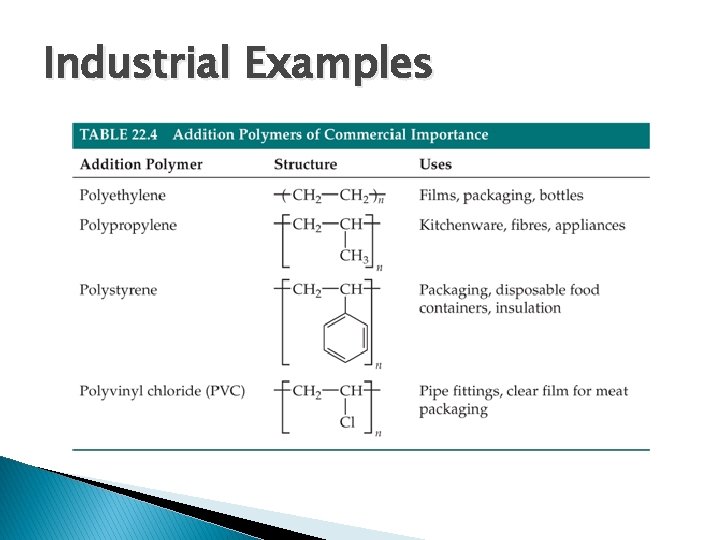
Industrial Examples
Physical Properties � Bonds allow atoms to move around making the molecular chain flexible � Macromolecules have different molecular weights ◦ Sometimes distributed evenly ◦ Sometimes concentrated in one area of chain �Causes polymers to soften over time (no sharp melting point) � Holding various chains together can make polymers harder, denser, less soluble, more resistant to heat
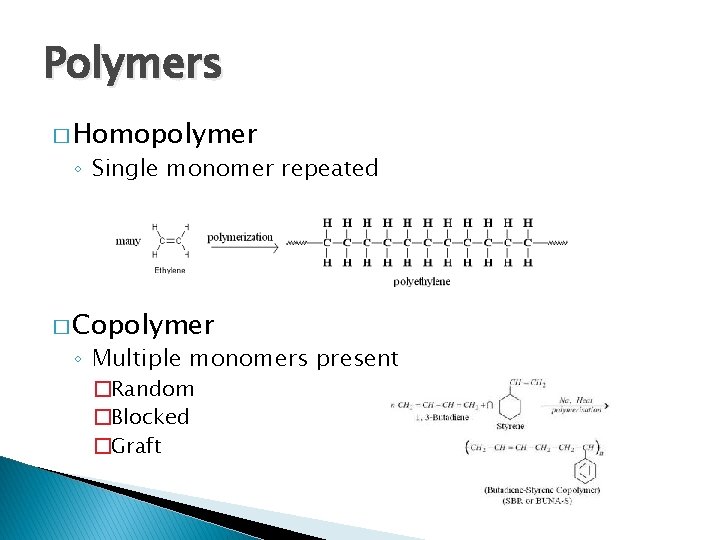
Polymers � Homopolymer ◦ Single monomer repeated � Copolymer ◦ Multiple monomers present �Random �Blocked �Graft
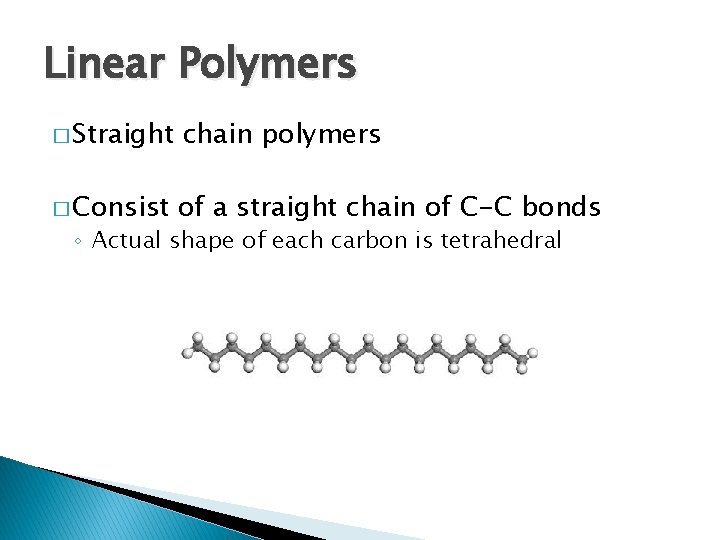
Linear Polymers � Straight chain polymers � Consist of a straight chain of C-C bonds ◦ Actual shape of each carbon is tetrahedral
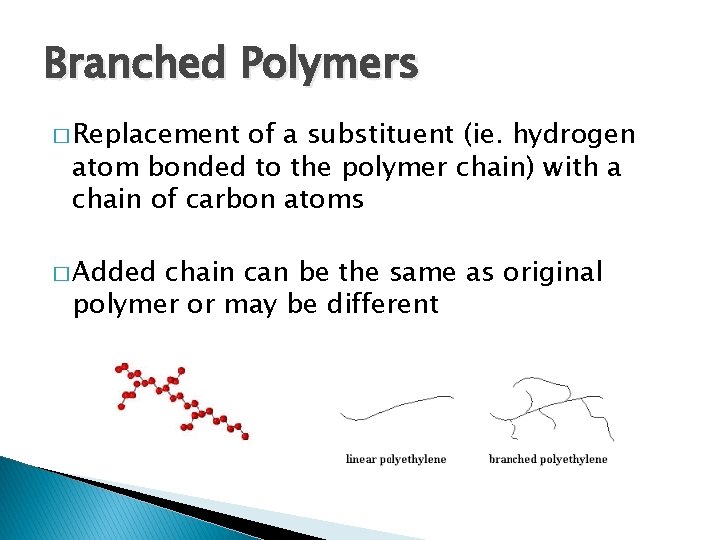
Branched Polymers � Replacement of a substituent (ie. hydrogen atom bonded to the polymer chain) with a chain of carbon atoms � Added chain can be the same as original polymer or may be different
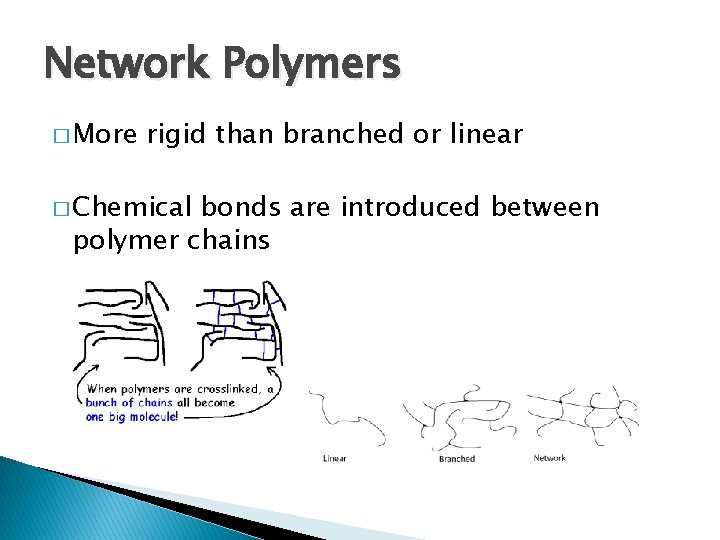
Network Polymers � More rigid than branched or linear � Chemical bonds are introduced between polymer chains

Cross-linking � Forming chains � More of chemical bonds between polymer cross-links = more rigid/stronger ◦ Plastic wrap vs. juice container
Plastics � Solid polymers that can be formed into various shapes by applying heat and pressure ◦ Thermoplastics ◦ Thermosetting plastic ◦ Elastomers
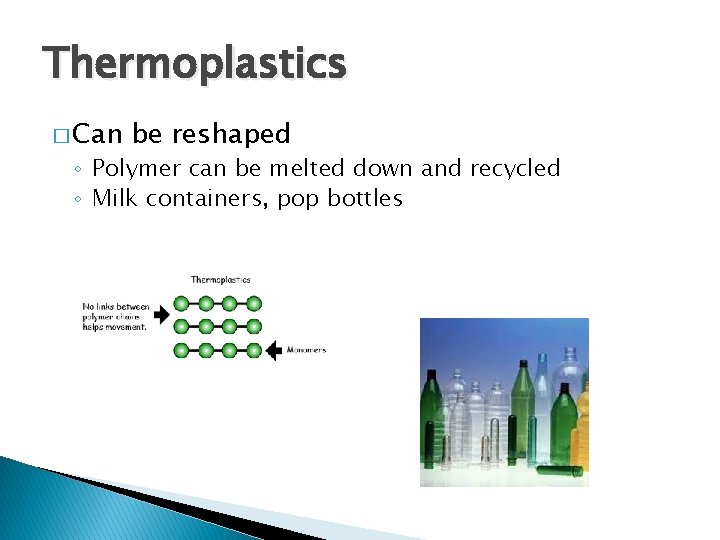
Thermoplastics � Can be reshaped ◦ Polymer can be melted down and recycled ◦ Milk containers, pop bottles
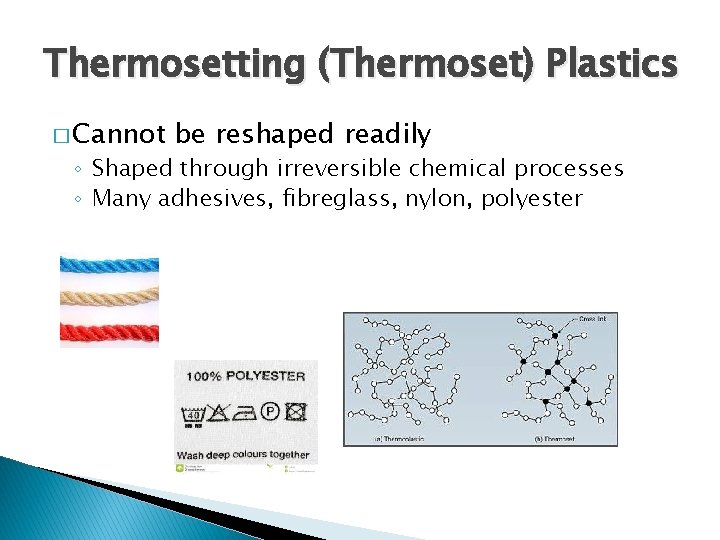
Thermosetting (Thermoset) Plastics � Cannot be reshaped readily ◦ Shaped through irreversible chemical processes ◦ Many adhesives, fibreglass, nylon, polyester

Thermoplastics Vs. Thermoset

Elastomers � Exhibit rubbery/elastic behaviour ◦ Regain shape after stretching or bending ◦ Crosslinking present but not as 3 D/close as thermoset ◦ Rubber

Polymers in Industry � Strength � Resistant and flexibility gives a variety of uses to oxidation � Reinforcing material can be added to make an even stronger product (ie. Fibreglass)
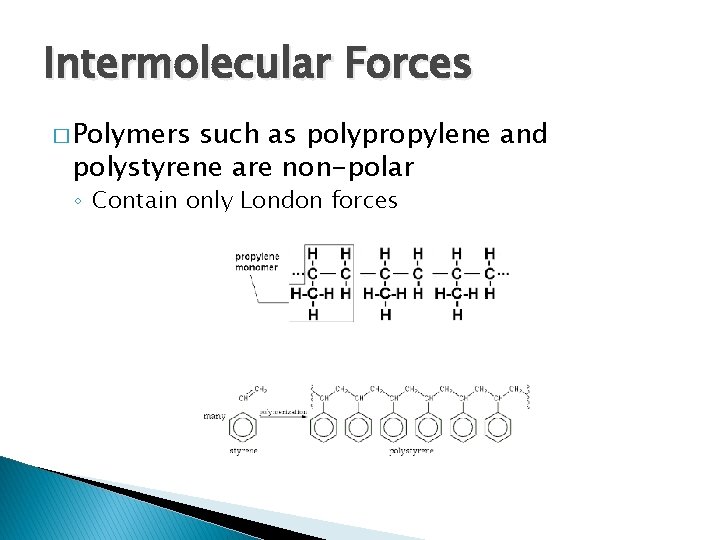
Intermolecular Forces � Polymers such as polypropylene and polystyrene are non-polar ◦ Contain only London forces
Intermolecular Forces � Most functional groups are polar, adding them makes the polymer polar ◦ With polarity brings in the possibility of Dipoledipole interactions and Hydrogen bonding ◦ More functional groups increases intermolecular forces
Intermolecular Forces � Polymers sometimes have weak intermolecular forces (London) but since they are so large there are many points of interaction � Chain entanglement � Polymers move slowly even when heated
Intermolecular Forces � Higher molecular weight means the polymer chain is longer ◦ Larger molecules have more points of interaction therefore more intermolecular forces � Remember from CH 1120, stronger/more intermolecular forces increases boiling and melting points
Distance of Separation � When chains are closer together they will interact more (more intermolecular forces) � Half the distance doubles the intermolecular force
Distance of Separation � High ◦ ◦ ◦ Density Polyethylene (HDPE) Mostly straight chains Packed tightly together More intermolecular forces Lawn furniture, fuel tanks Strength more important than flexibility

Distance of Separation � Low ◦ ◦ ◦ Density Polyethylene (LDPE) Branched chains Cannot pack as tightly as HDPE Fewer intermolecular forces Plastic wrap, grocery bags Flexibility more important than strength
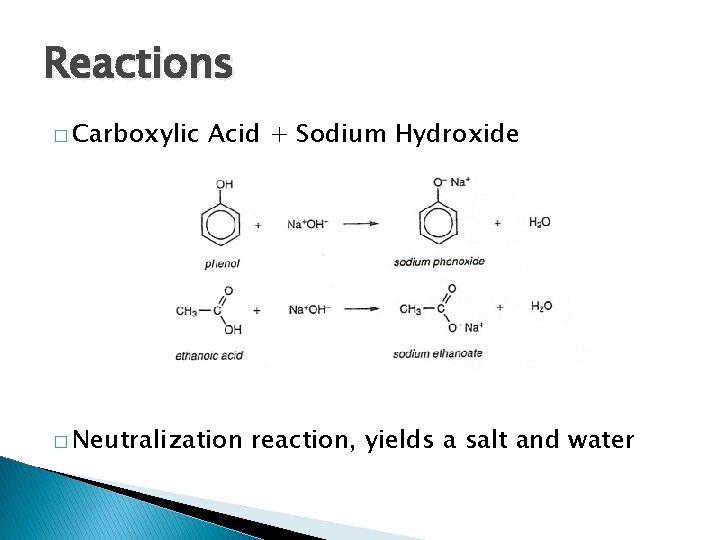
Reactions � Carboxylic Acid + Sodium Hydroxide � Neutralization reaction, yields a salt and water
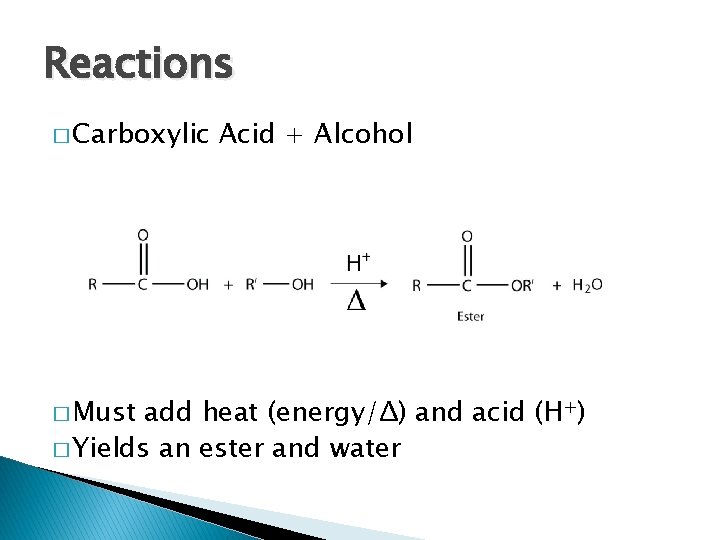
Reactions � Carboxylic � Must Acid + Alcohol add heat (energy/Δ) and acid (H+) � Yields an ester and water
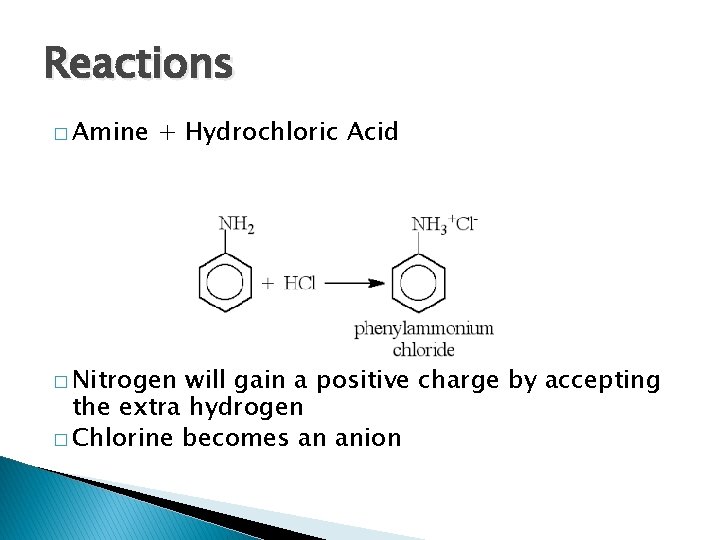
Reactions � Amine + Hydrochloric Acid � Nitrogen will gain a positive charge by accepting the extra hydrogen � Chlorine becomes an anion
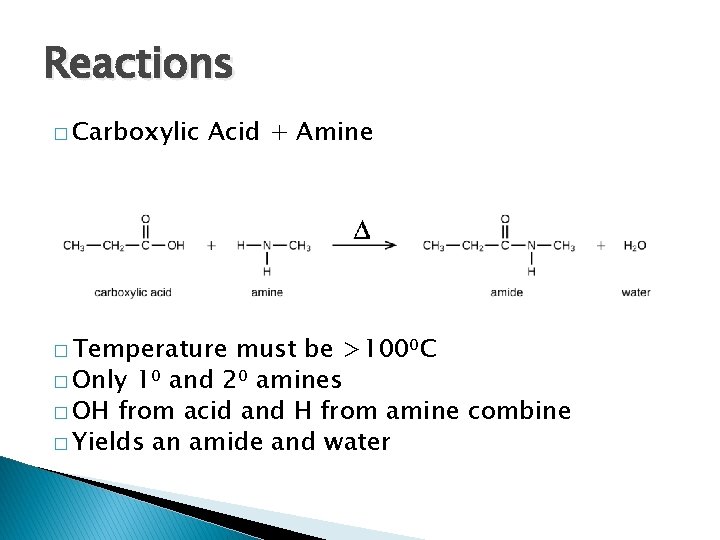
Reactions � Carboxylic Acid + Amine � Temperature must be >100⁰C � Only 1⁰ and 2⁰ amines � OH from acid and H from amine combine � Yields an amide and water

Polymerization � Always the same steps: 1. Initiation 2. Propagation 3. Termination � Two types 1. Free radical addition polymerization �C=C is broken 2. Condensation polymerization �Water is removed
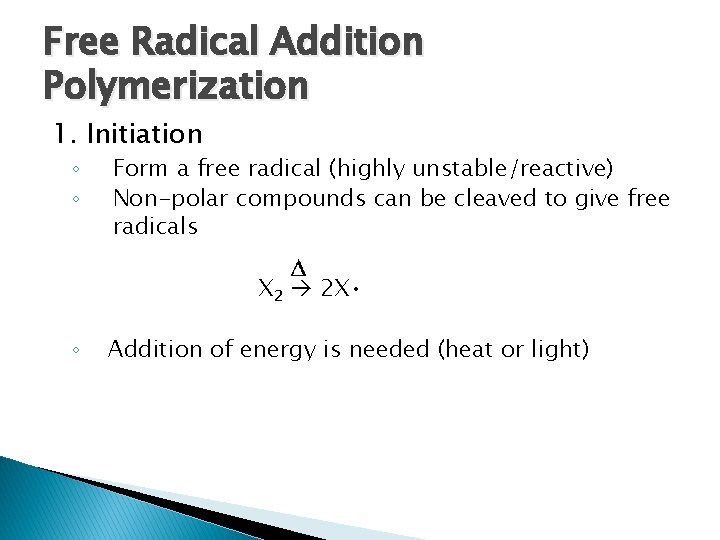
Free Radical Addition Polymerization 1. Initiation ◦ ◦ Form a free radical (highly unstable/reactive) Non-polar compounds can be cleaved to give free radicals X 2 2 X • ◦ Addition of energy is needed (heat or light)
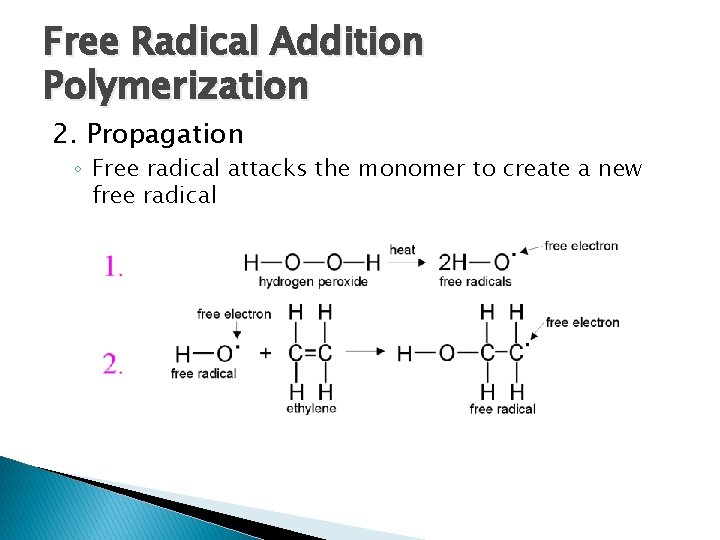
Free Radical Addition Polymerization 2. Propagation ◦ Free radical attacks the monomer to create a new free radical
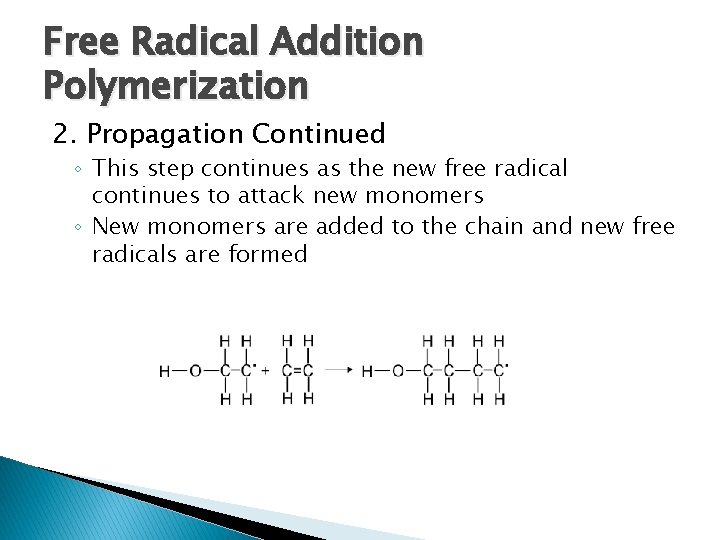
Free Radical Addition Polymerization 2. Propagation Continued ◦ This step continues as the new free radical continues to attack new monomers ◦ New monomers are added to the chain and new free radicals are formed
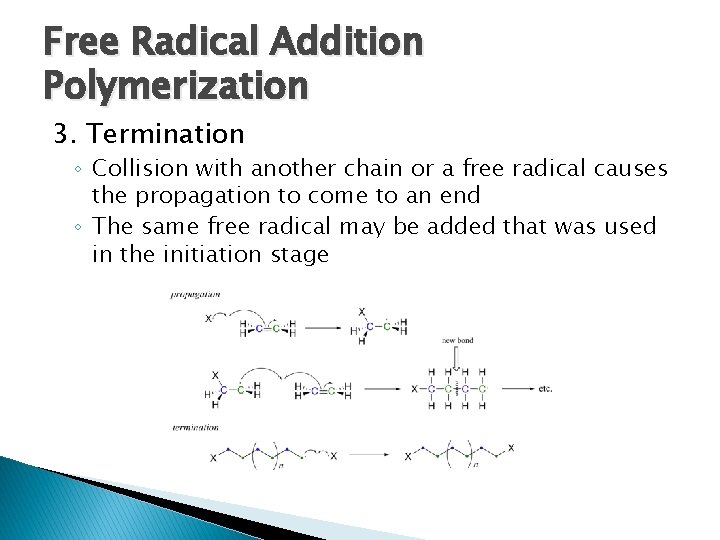
Free Radical Addition Polymerization 3. Termination ◦ Collision with another chain or a free radical causes the propagation to come to an end ◦ The same free radical may be added that was used in the initiation stage

Free Radical Addition Polymerization � Know the mechanisms to create: ◦ Polypropylene ◦ Polystyrene ◦ Polyvinyl chloride (PVC)
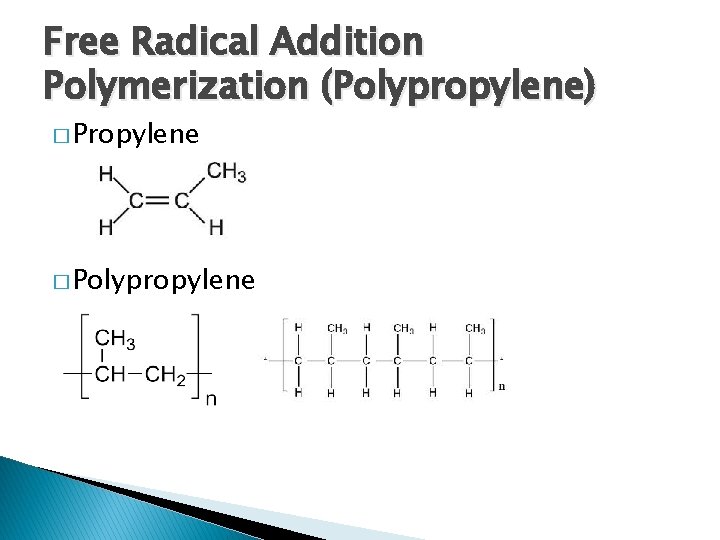
Free Radical Addition Polymerization (Polypropylene) � Propylene � Polypropylene
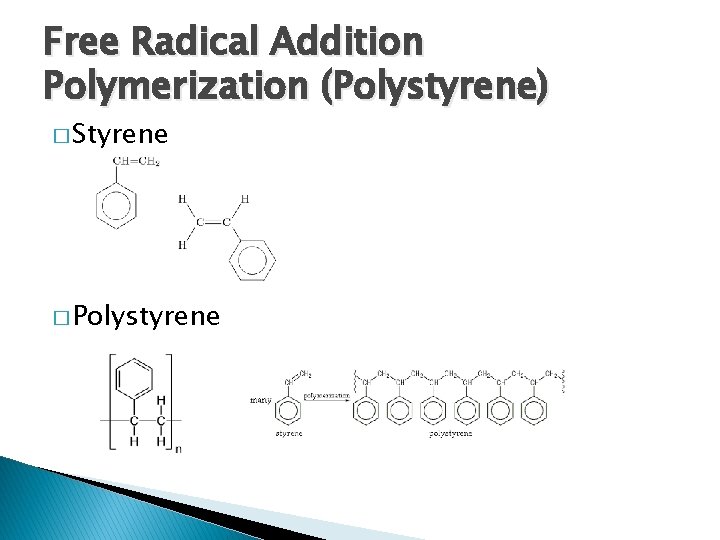
Free Radical Addition Polymerization (Polystyrene) � Styrene � Polystyrene
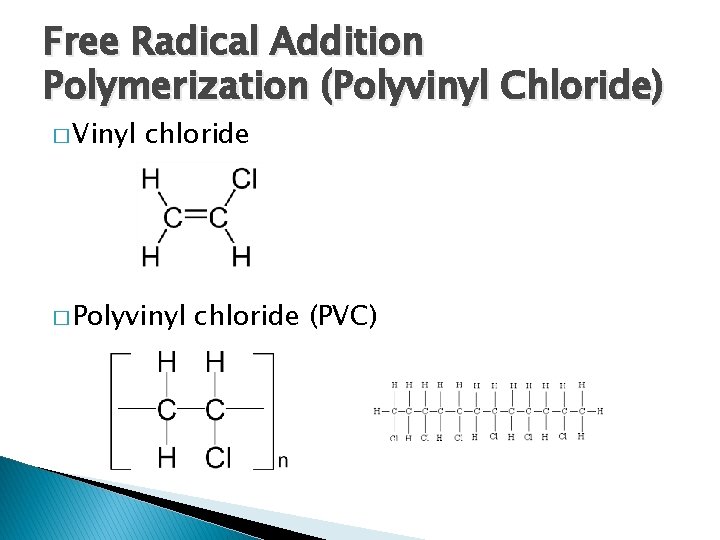
Free Radical Addition Polymerization (Polyvinyl Chloride) � Vinyl chloride � Polyvinyl chloride (PVC)
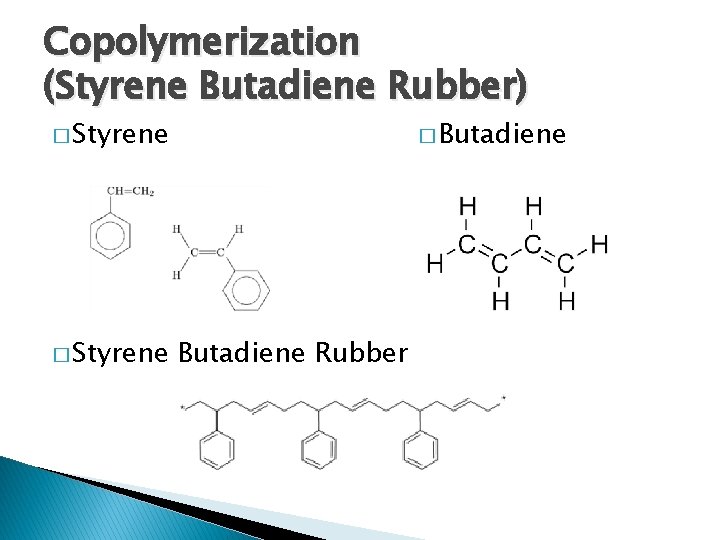
Copolymerization (Styrene Butadiene Rubber) � Butadiene � Styrene Butadiene Rubber
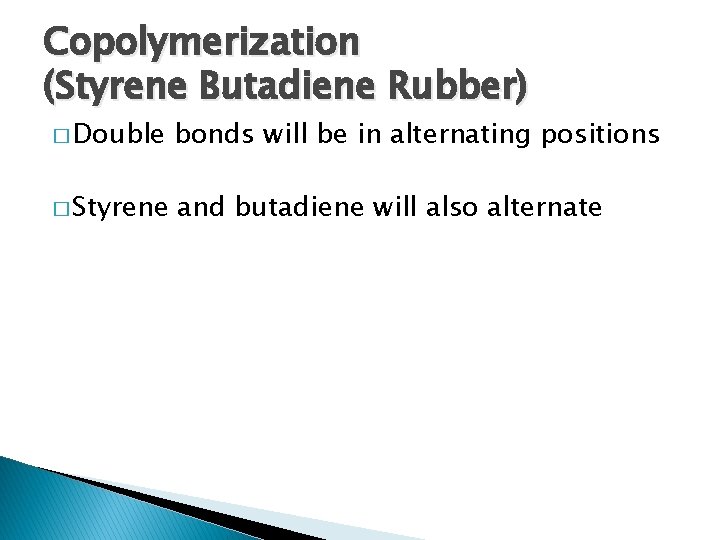
Copolymerization (Styrene Butadiene Rubber) � Double bonds will be in alternating positions � Styrene and butadiene will also alternate
Condensation Polymerization � As the polymer grows water is released as a product
Condensation Polymerization � Two types: 1. Polyester Formation �Dicarboxylic Acid + Dialcohol Polyester+ Water �-OH is lost from acid, -H from alcohol to make H 2 O �Initiation: requires heat and strong acid �Termination: cool and neutralize 2. Polyamide Formation �Dicarboxylic Acid + Diamine Polyamide + Water �-OH is lost from acid, -H from amine to make H 2 O �Initiation: heat above 100⁰C �Termination: cool below 100⁰C
Condensation Polymerization (Polyester) � Dicarboxylic � Acid + Dialcohol Polyester + Water and heat are required for reaction � Termination neutralizing is carried out by cooling and
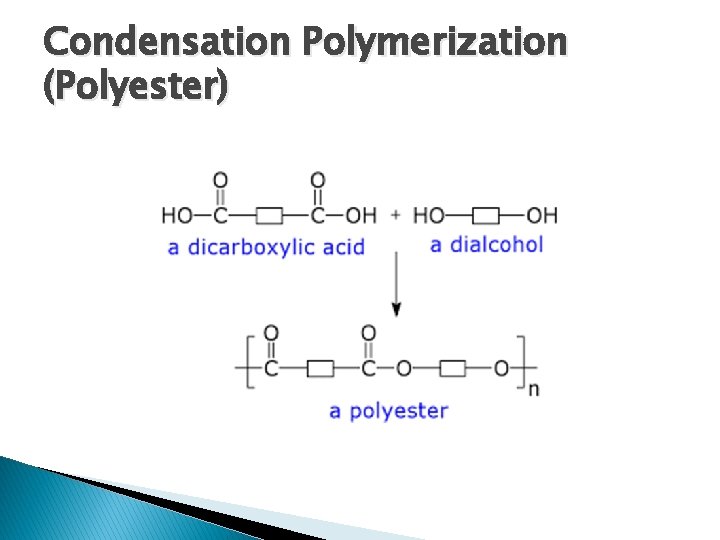
Condensation Polymerization (Polyester)
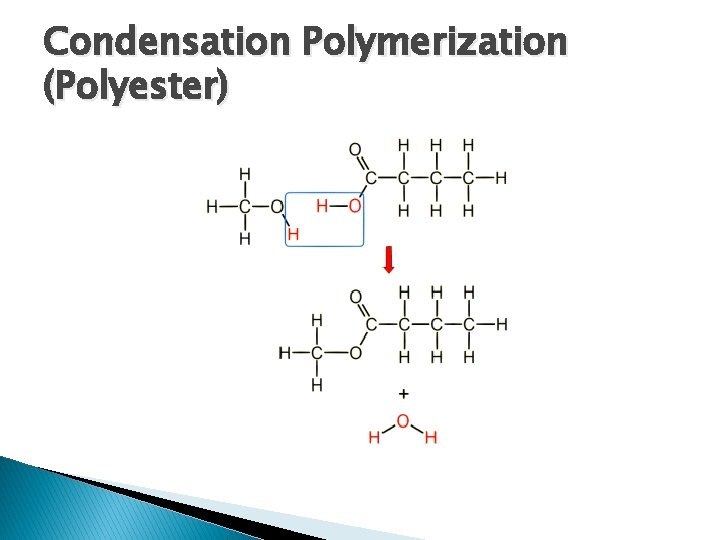
Condensation Polymerization (Polyester)
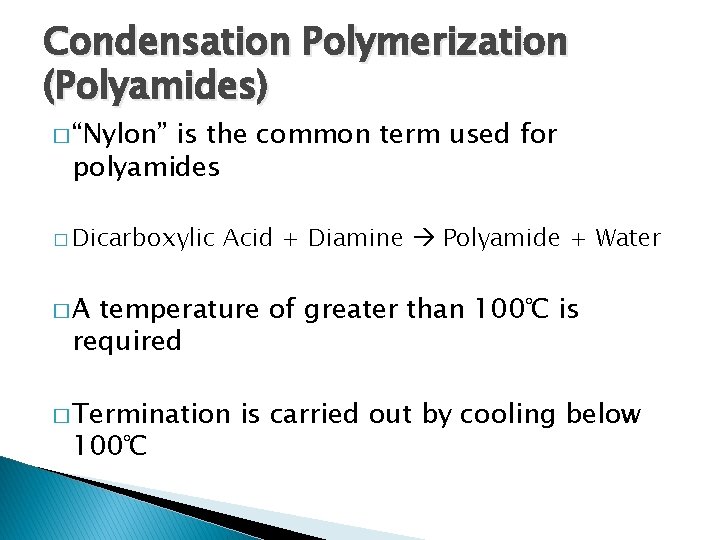
Condensation Polymerization (Polyamides) � “Nylon” is the common term used for polyamides � Dicarboxylic Acid + Diamine Polyamide + Water �A temperature of greater than 100℃ is required � Termination 100℃ is carried out by cooling below
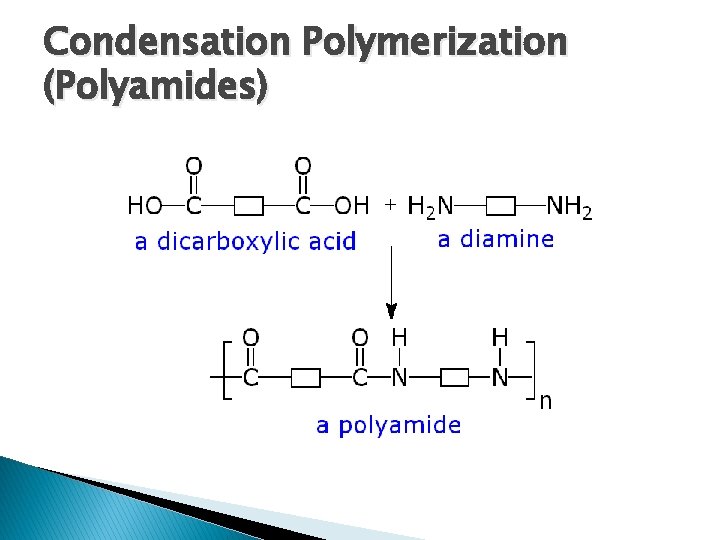
Condensation Polymerization (Polyamides)
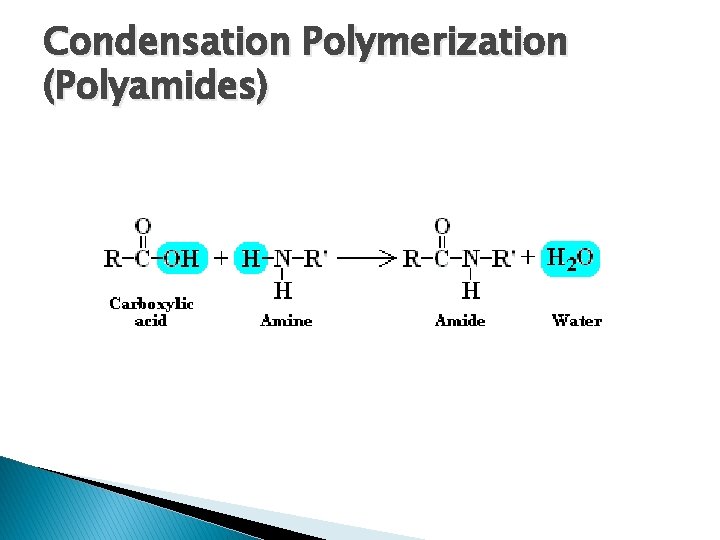
Condensation Polymerization (Polyamides)

Formation of Acetate � Cellulose + Acetic Acid Acetate � Cellulose is already a natural polymer, we alter it with acetic acid � Sulfuric reaction acid and heat are needed to speed up
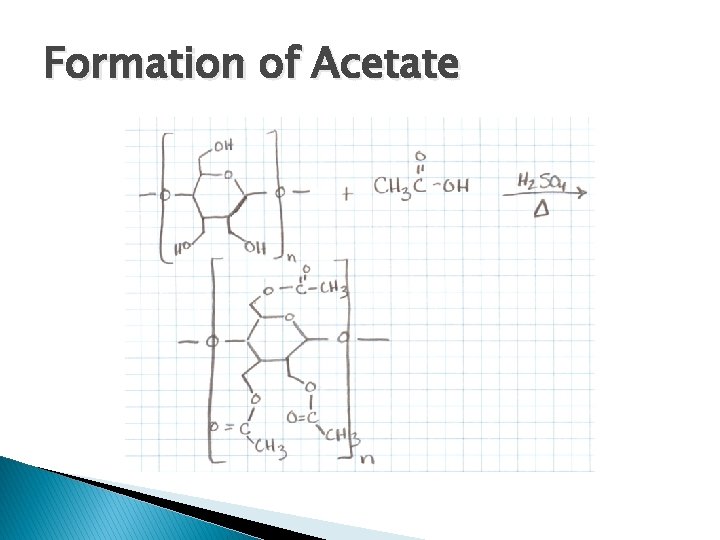
Formation of Acetate
Formation of Rayon � Cellulose + Na. OH + CS 2 Rayon is a naturally occurring polymer, in this case we alter it by adding sodium hydroxide and carbon disulfide
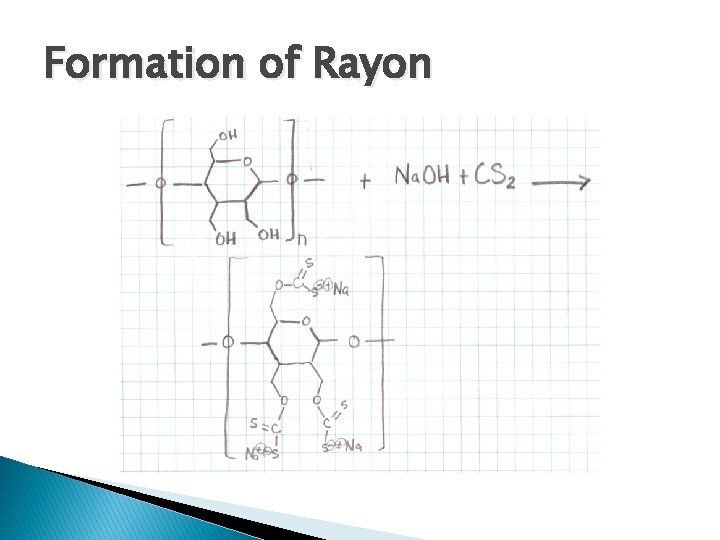
Formation of Rayon
- Slides: 73