Unit 1 Notes Biological Elements Biomolecules 1 Atoms
Unit 1 Notes: Biological Elements & Biomolecules

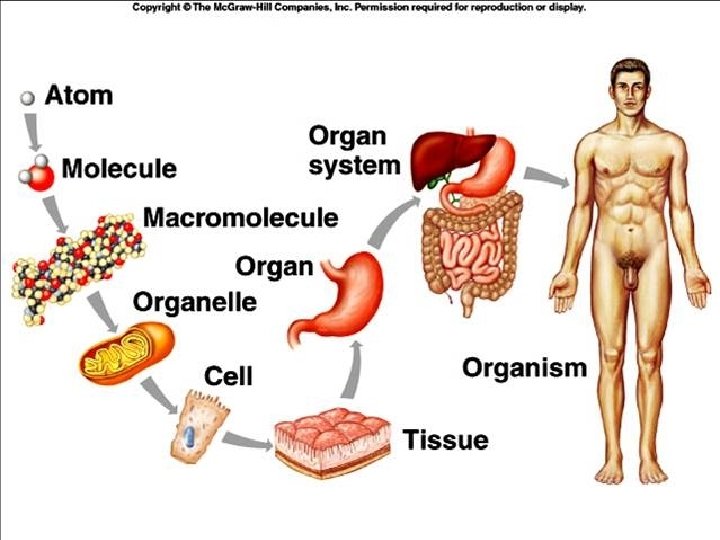
(1) Atoms, Energy & Living Things • All living things are made of atoms: – Missing valence electrons drive all chemical reactions inside living things. – Atoms bond together to gain full valence shells and become stable. • All living things require energy in order to complete life processes.
(2) Biological Elements • CHONPS (Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus, Sulfur) • These 6 elements are found in all living things. • Carbon bonds form the framework for all major molecules found in living systems.

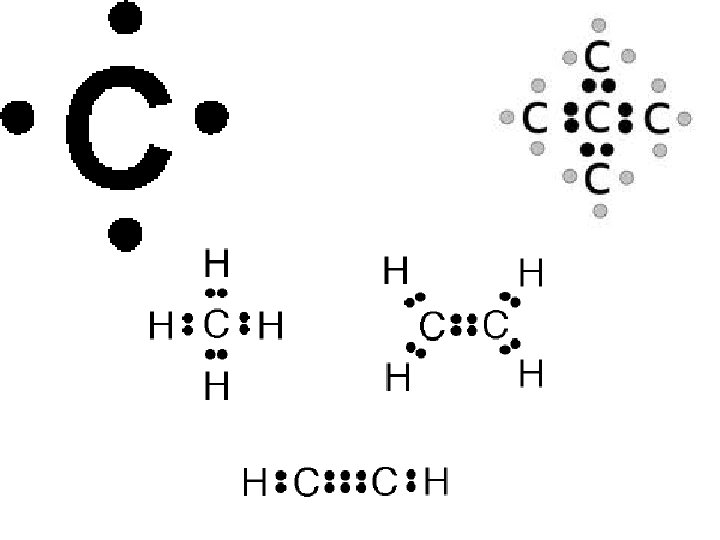
(3) Why is Carbon So Special? • Carbon has 4 valence electrons. • Carbon can form up to four bonds with other atoms. • This allows Carbon to form lots of different types of structures and molecules, all with different functions.
(4) The Biomolecules • • Carbohydrates (Carbs) Lipids (Fats) Protein Nucleic Acids • These are the 4 molecules that make up all living things, each composed of CHONPS.
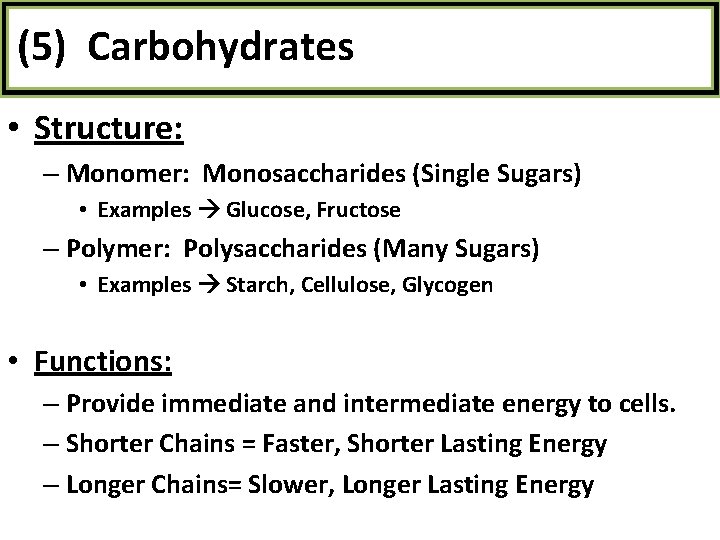
(5) Carbohydrates • Structure: – Monomer: Monosaccharides (Single Sugars) • Examples Glucose, Fructose – Polymer: Polysaccharides (Many Sugars) • Examples Starch, Cellulose, Glycogen • Functions: – Provide immediate and intermediate energy to cells. – Shorter Chains = Faster, Shorter Lasting Energy – Longer Chains= Slower, Longer Lasting Energy

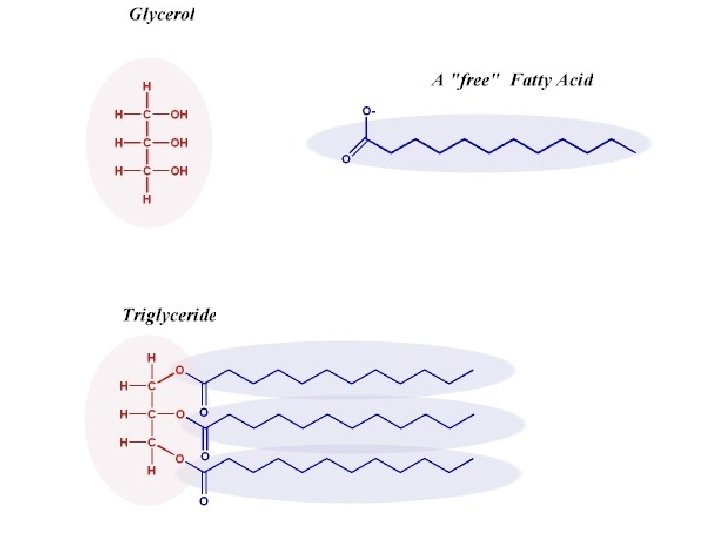
(6) Lipids • Structure: – Monomer: Fatty Acids and Glycerols – Polymer: Lipid • Examples Phospholipids, Oils, Cholesterol, Triglycerides • Functions: – Provide long term energy storage to cells. – Phospholipids form protective cell membranes.

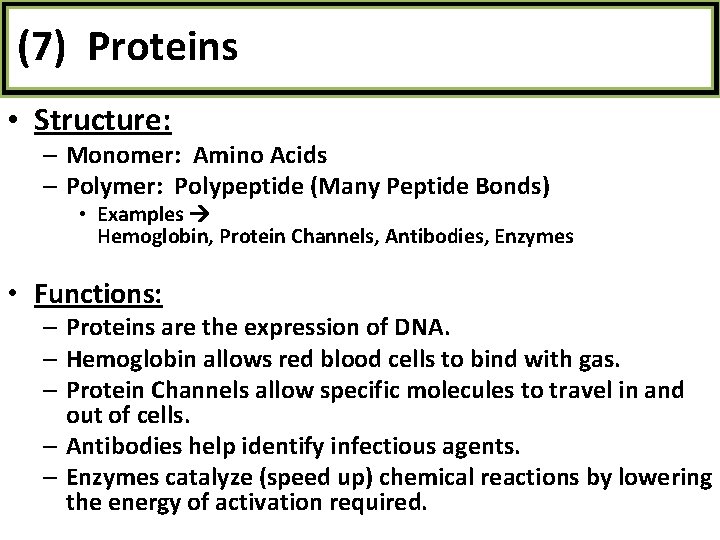
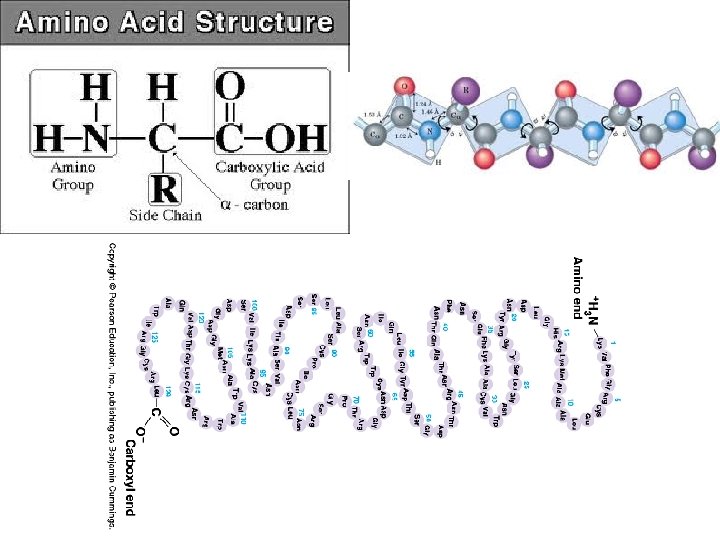
(7) Proteins • Structure: – Monomer: Amino Acids – Polymer: Polypeptide (Many Peptide Bonds) • Examples Hemoglobin, Protein Channels, Antibodies, Enzymes • Functions: – Proteins are the expression of DNA. – Hemoglobin allows red blood cells to bind with gas. – Protein Channels allow specific molecules to travel in and out of cells. – Antibodies help identify infectious agents. – Enzymes catalyze (speed up) chemical reactions by lowering the energy of activation required.

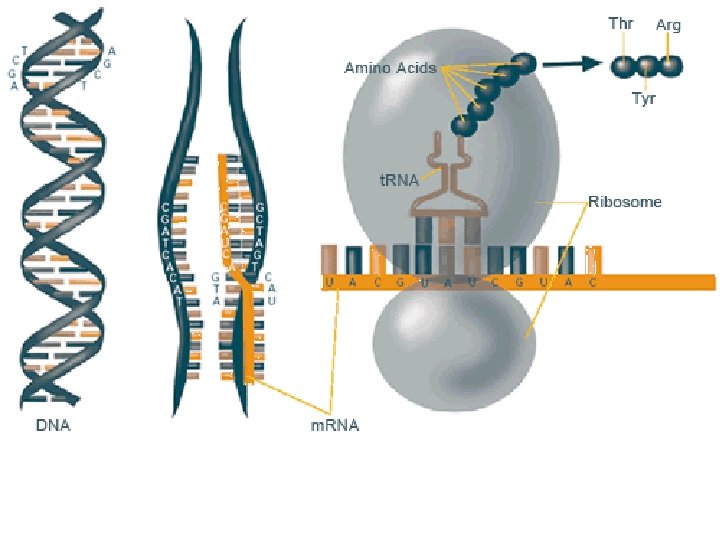
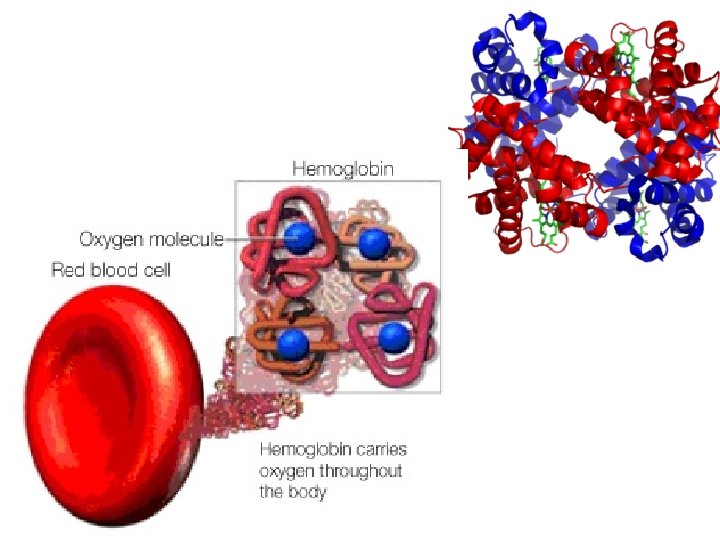
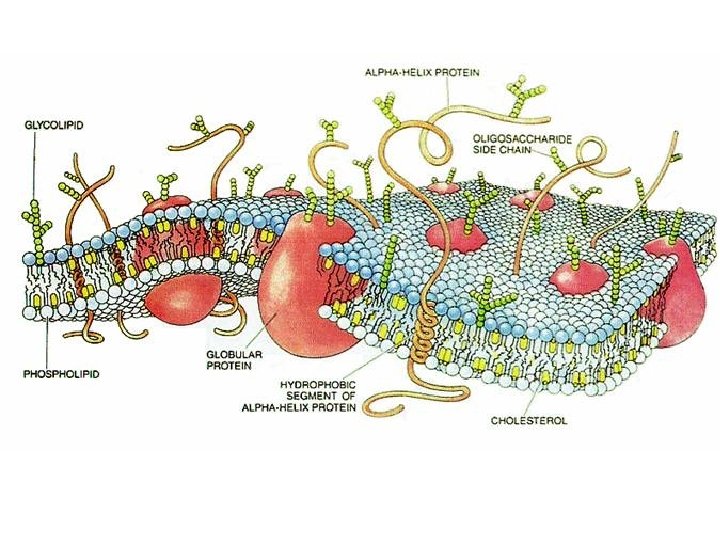
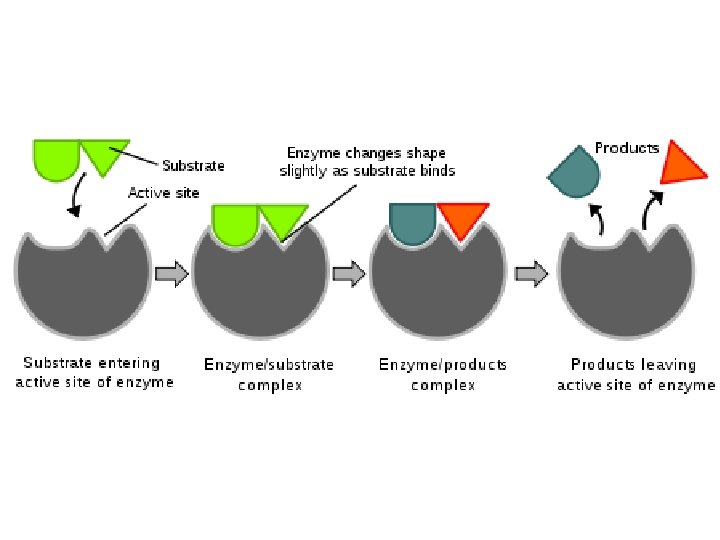
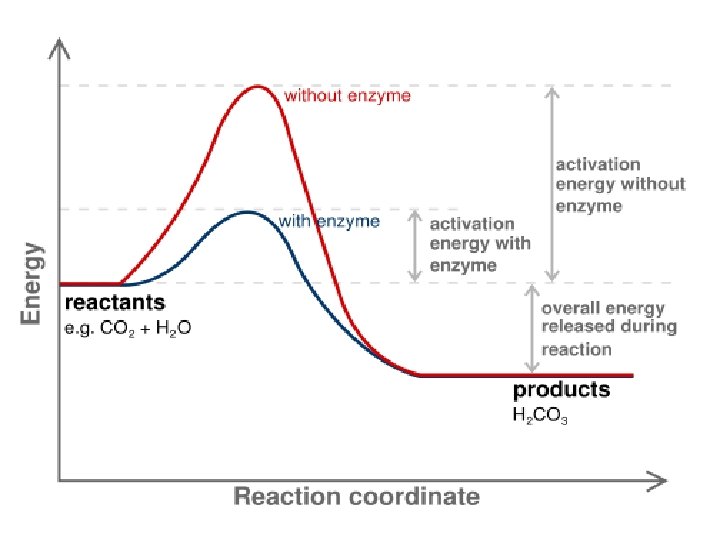
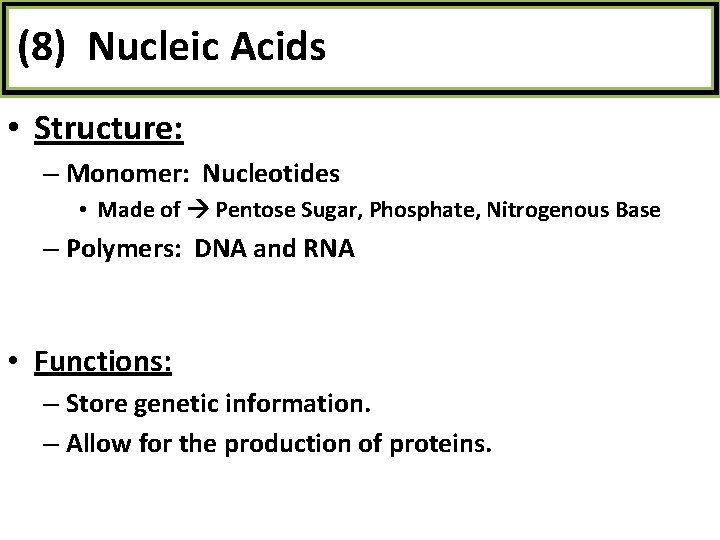
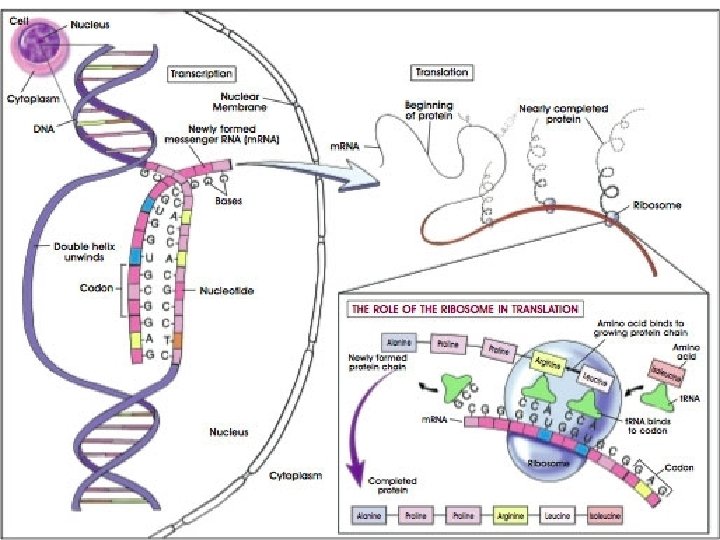
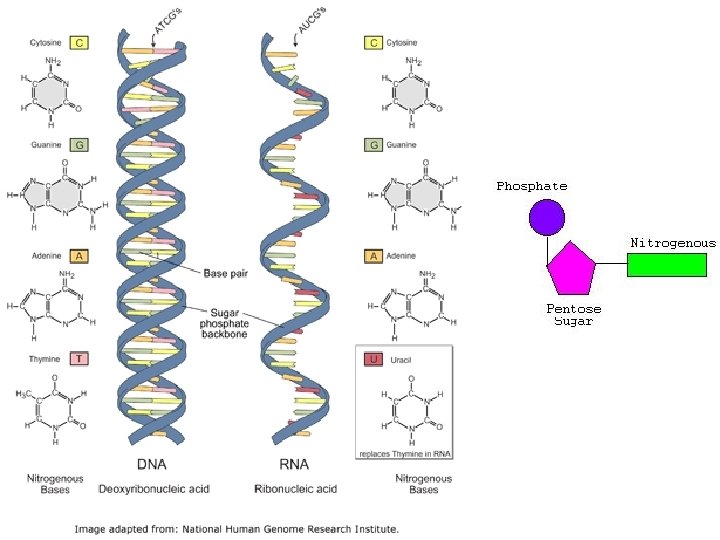
(8) Nucleic Acids • Structure: – Monomer: Nucleotides • Made of Pentose Sugar, Phosphate, Nitrogenous Base – Polymers: DNA and RNA • Functions: – Store genetic information. – Allow for the production of proteins.
- Slides: 24